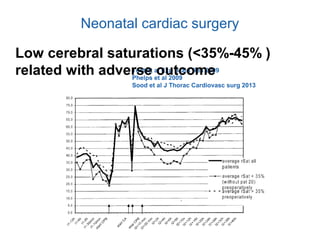
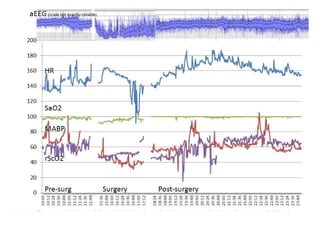
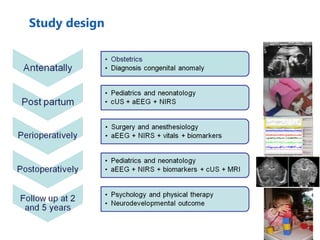
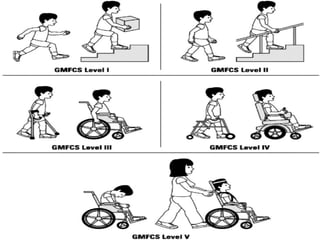
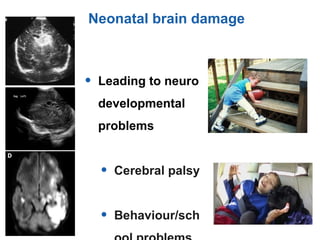
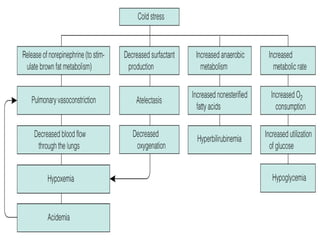
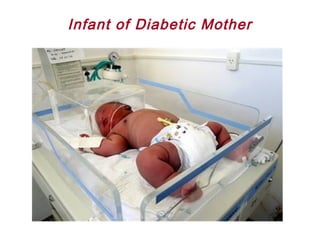
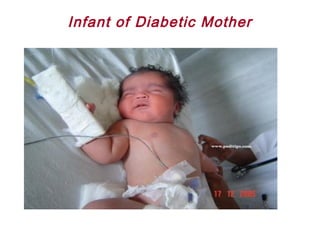
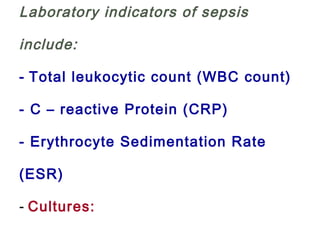
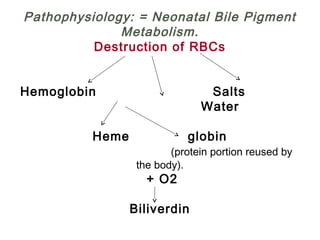
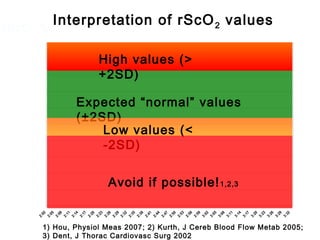
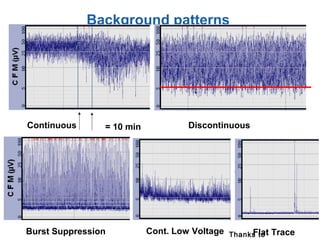
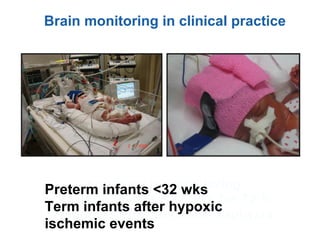
This document discusses several topics related to high-risk neonates and their neurodevelopmental outcomes. It begins by defining high-risk neonates as babies exposed to conditions that endanger their survival. Some factors that can contribute to high-risk status include high-risk pregnancies, medical illnesses in the mother, complications during labor, and neonatal medical conditions. The document then discusses several conditions in more detail, including hypothermia, hyperthermia, hypoglycemia, infants of diabetic mothers, and neonatal sepsis. It provides definitions, risk factors, clinical presentations, and management strategies for each of these conditions.







































































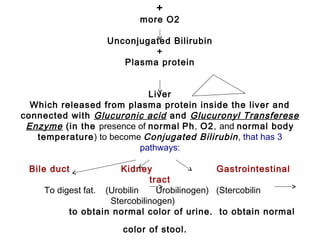
























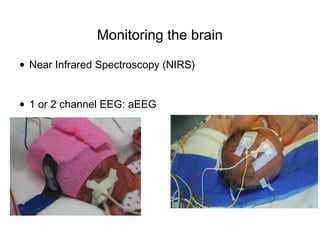
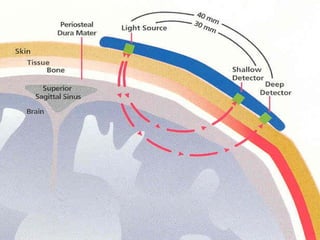
![Near Infrared Spectroscopy (NIRS)
• Monitoring technique for cerebral oxygenation and
haemodynamics
• Based on absorption of near-infrared light by
oxygenated [O2Hb] and deoxygenated Hb [HHb]
• Absorption-changes in NIR-light (∆ ODs) can be
converted in changes of [∆O2Hb] and [∆HHb]
• Regional (mixed) cerebral O2-saturation: rScO2](https://image.slidesharecdn.com/high-riskneonate-141114084056-conversion-gate02/85/High-risk-neonate-116-320.jpg)
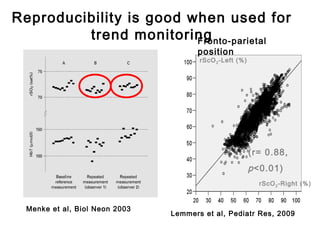



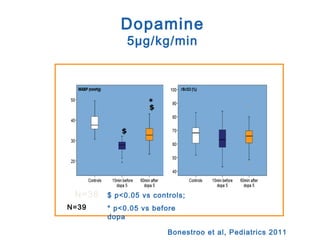
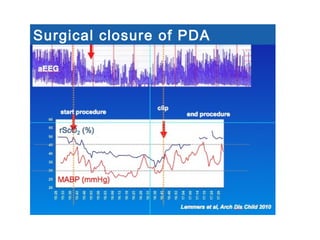
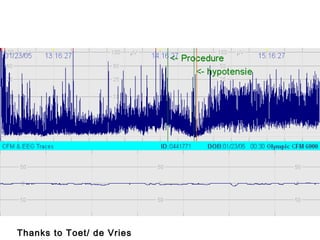
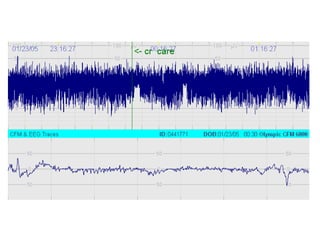
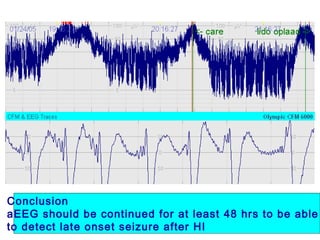

![*
p<0.05 vs pre-clip
surger
y
GA 26.7 ±1.8 wks
PNA 7 days [4-39]
PDA surgery after failure medication](https://image.slidesharecdn.com/high-riskneonate-141114084056-conversion-gate02/85/High-risk-neonate-136-320.jpg)