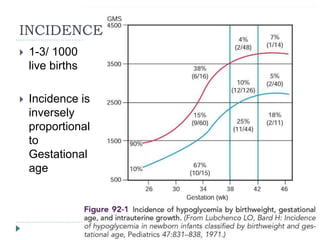
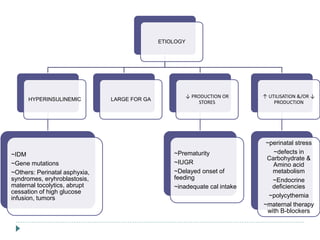
1) Hypoglycemia is common in newborns, affecting 1-3 per 1000 live births, with risk decreasing with increasing gestational age.
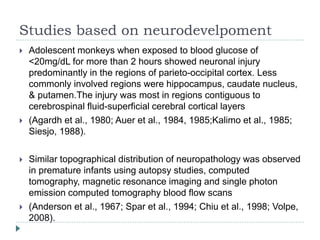
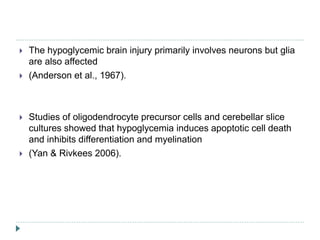
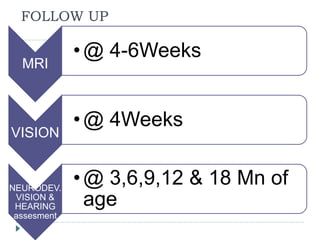
2) Significant neurodevelopmental deficits can occur in neonates who experience prolonged hypoglycemia. Studies in monkeys and infants show neuronal injury, particularly in parieto-occipital cortex and other regions.
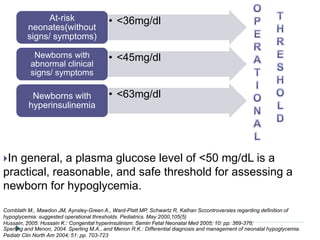
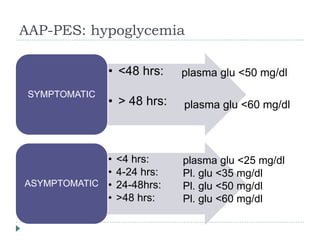
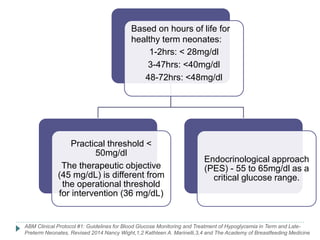
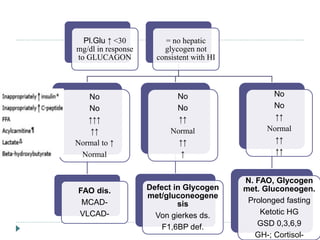
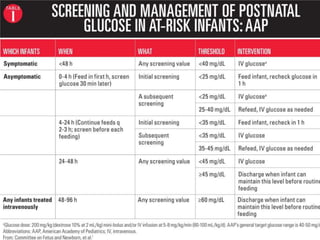
3) There is no consensus on the definition and threshold for treating hypoglycemia. Guidelines generally recommend treating if blood glucose is less than 50 mg/dL in symptomatic newborns or less than 36 mg/dL in at-risk but asymptomatic newborns.