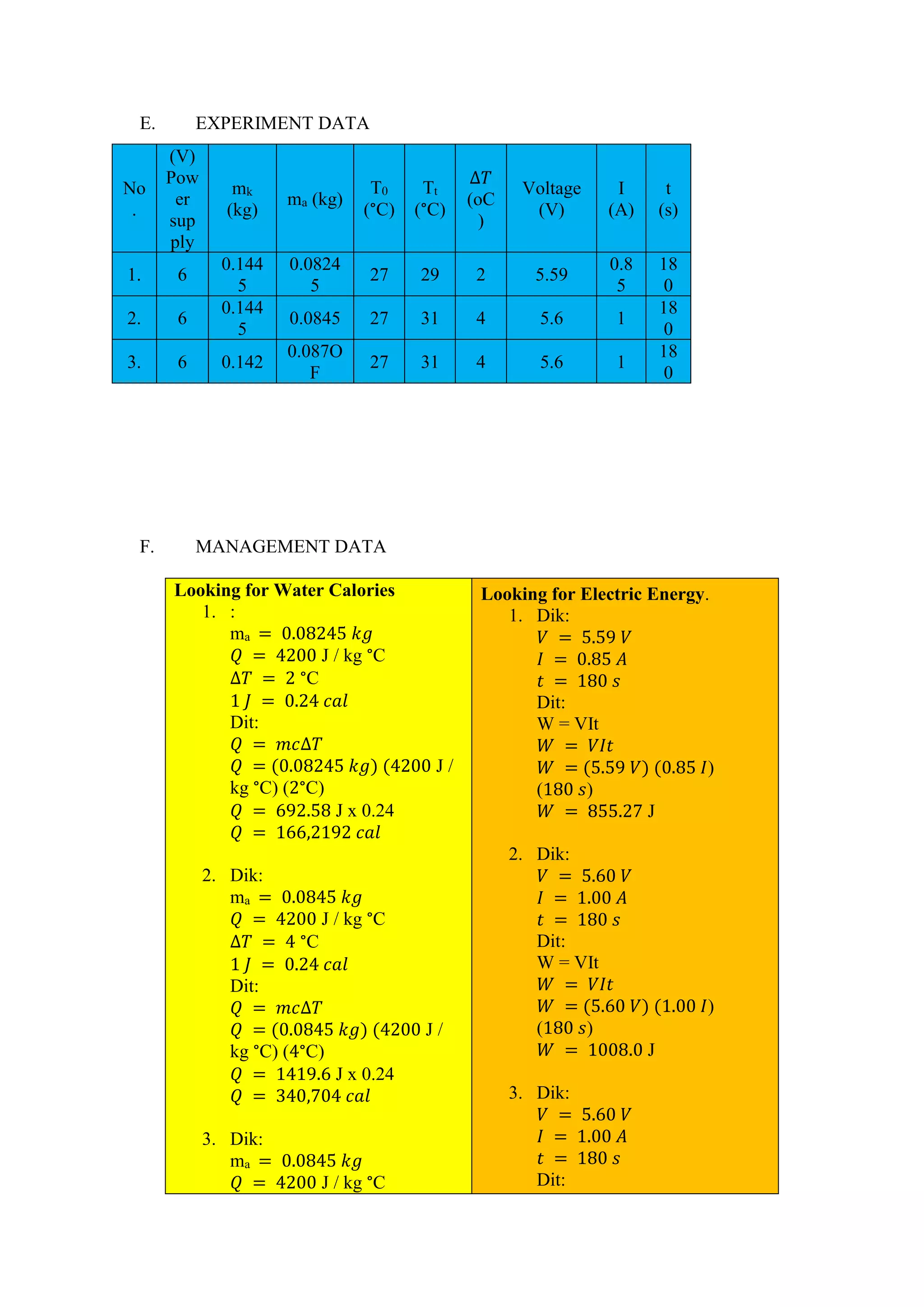
This document summarizes a practicum report on using a calorimeter to measure heat. The report details the purpose, theory, tools, steps, data, calculations, and discussion. The practicum aims to determine the type of calorimeter, amount of heat energy released, electrical energy received, and understand calorimeter material. Measurements are taken of the calorimeter's mass, temperature changes when electrical current is passed through. Calculations are done to find the heat, electrical energy, power, and verify the relationship between electrical and heat energy using a correlation factor.