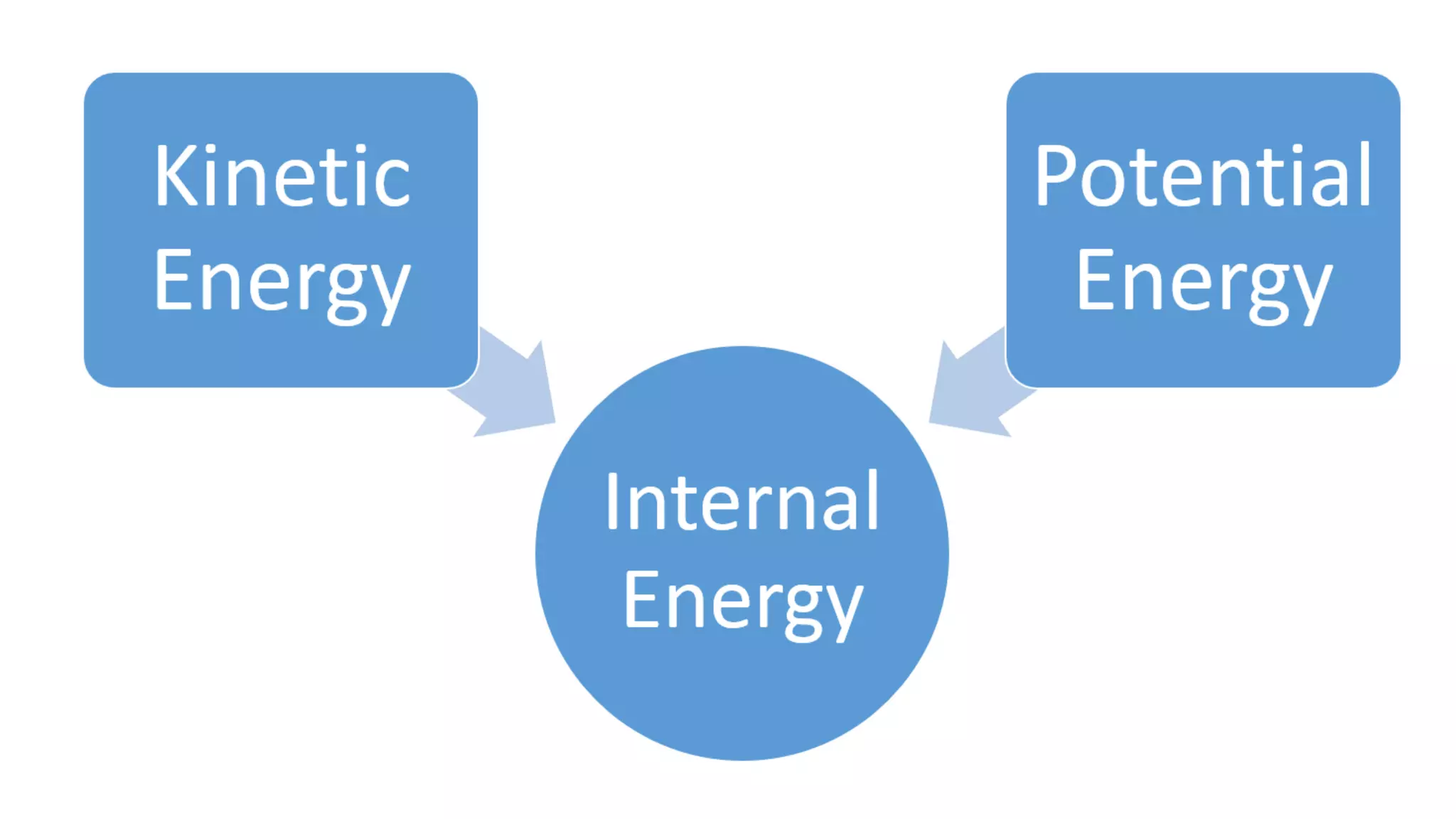
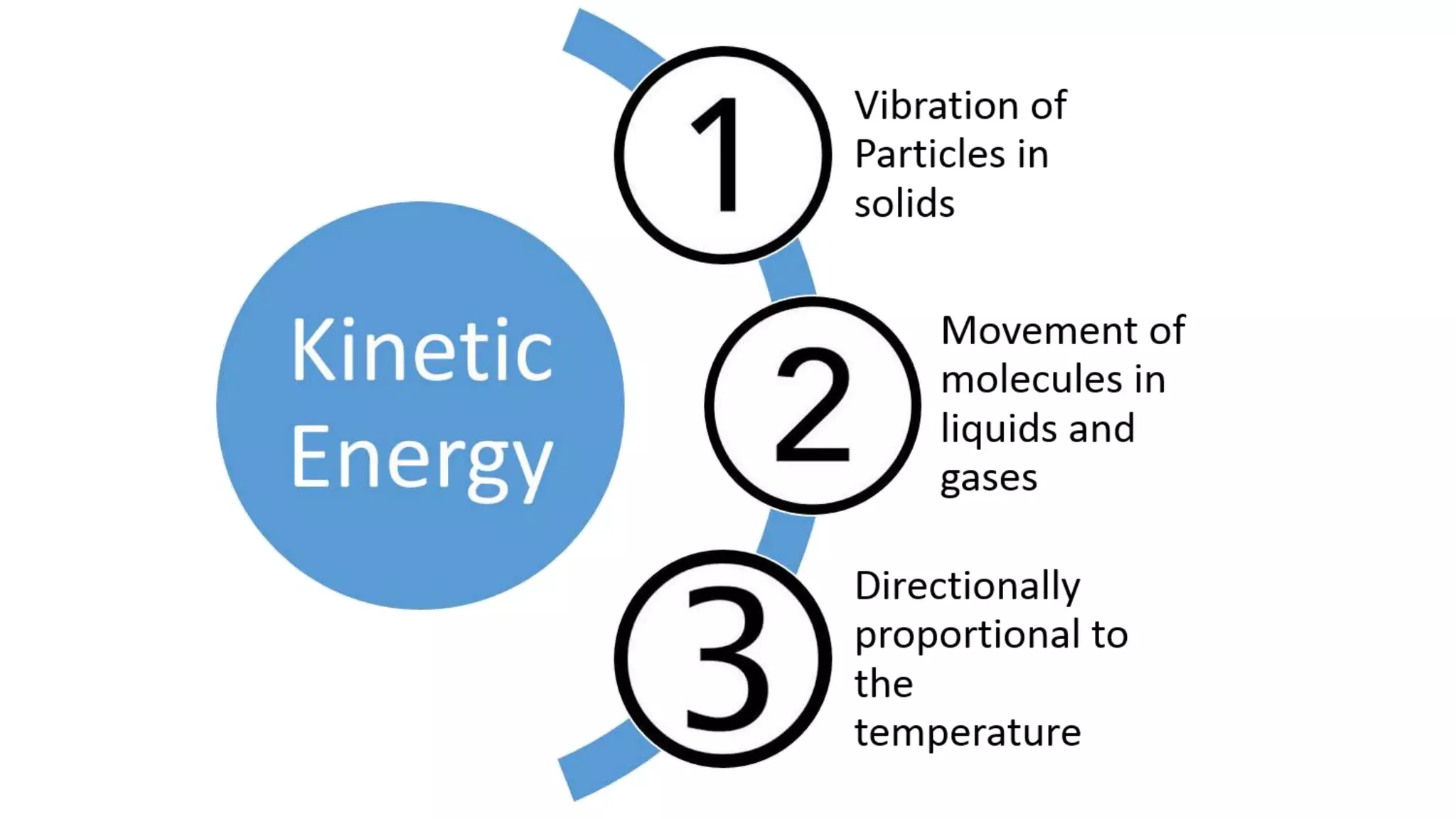
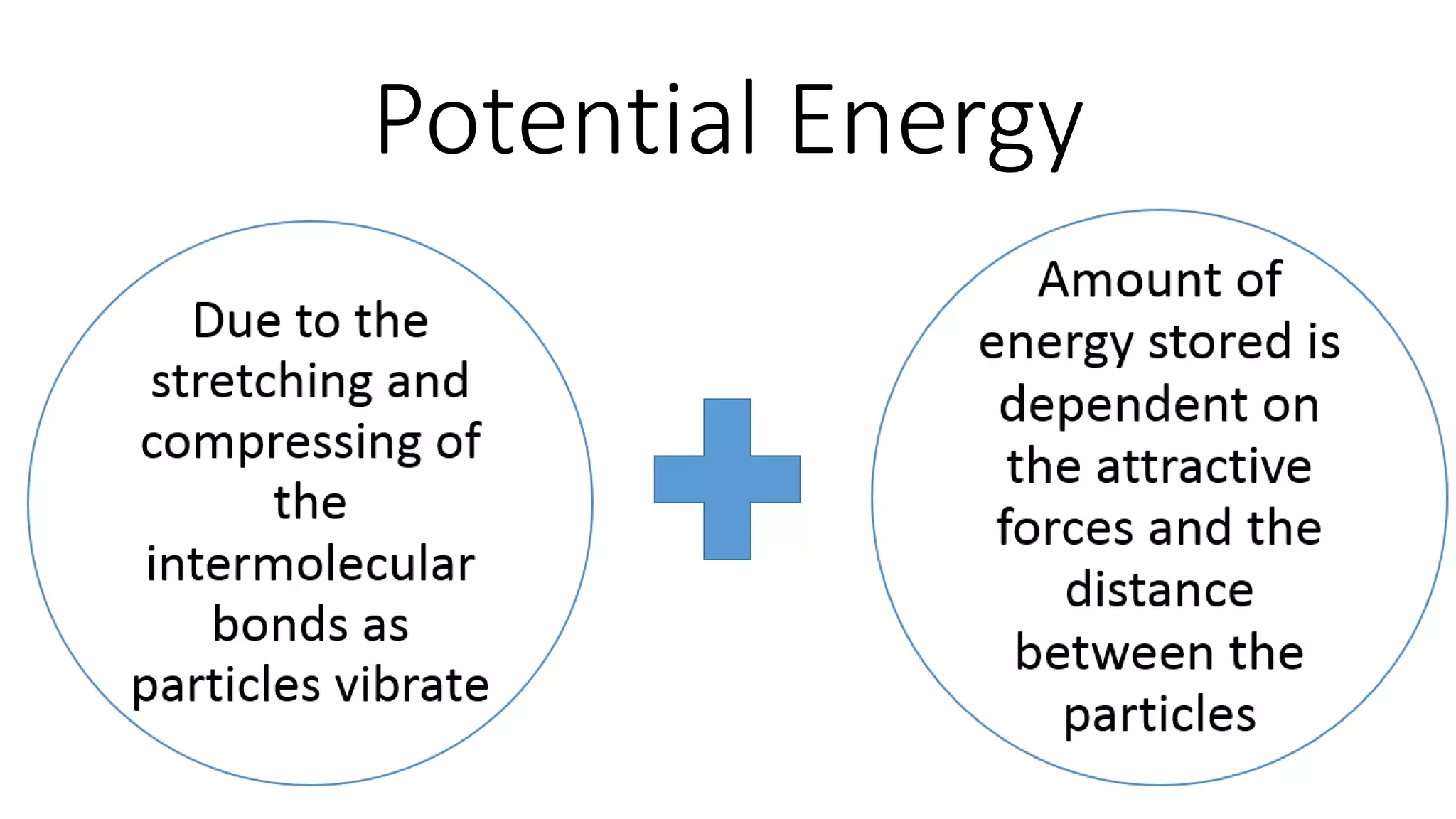
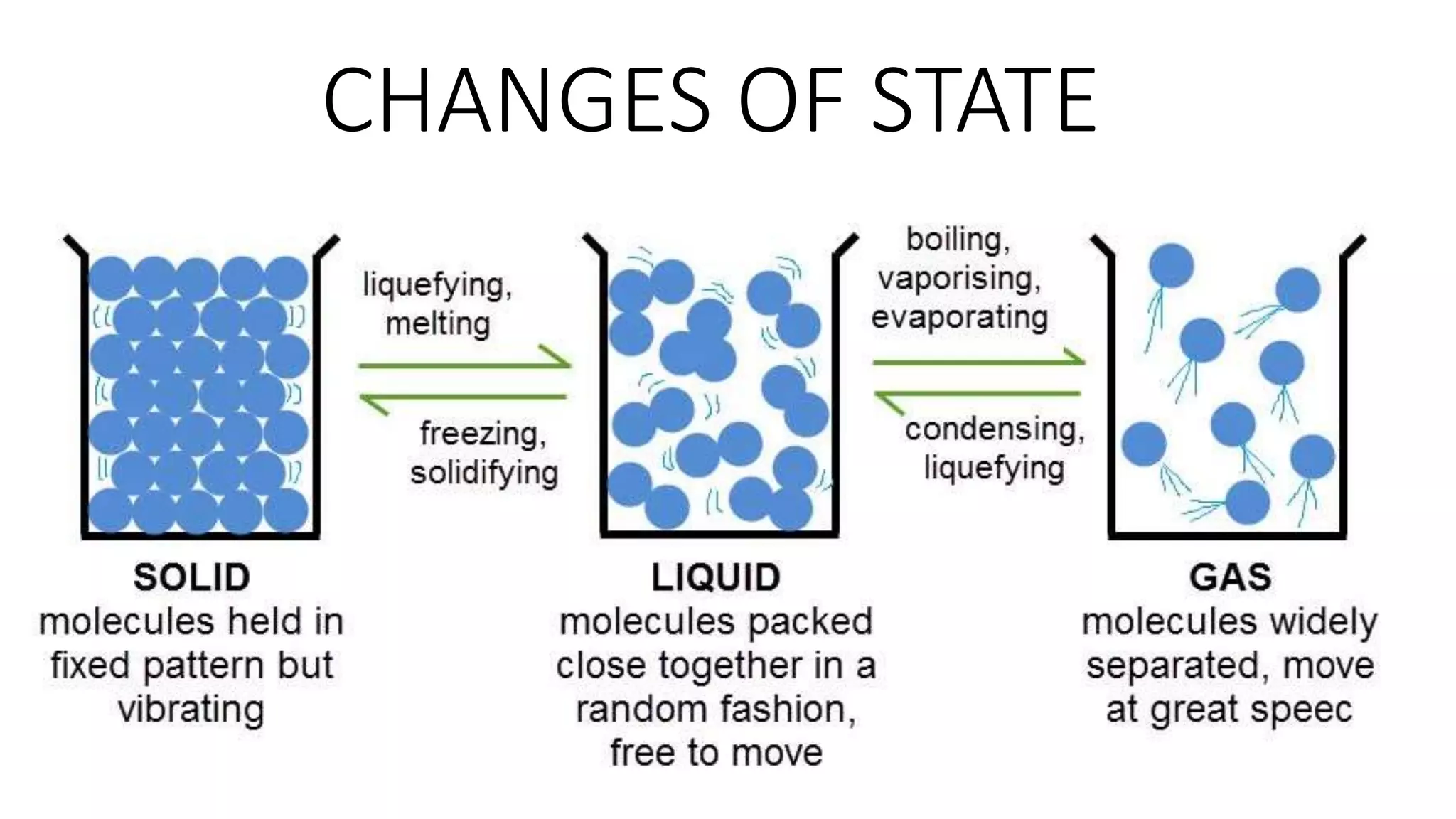
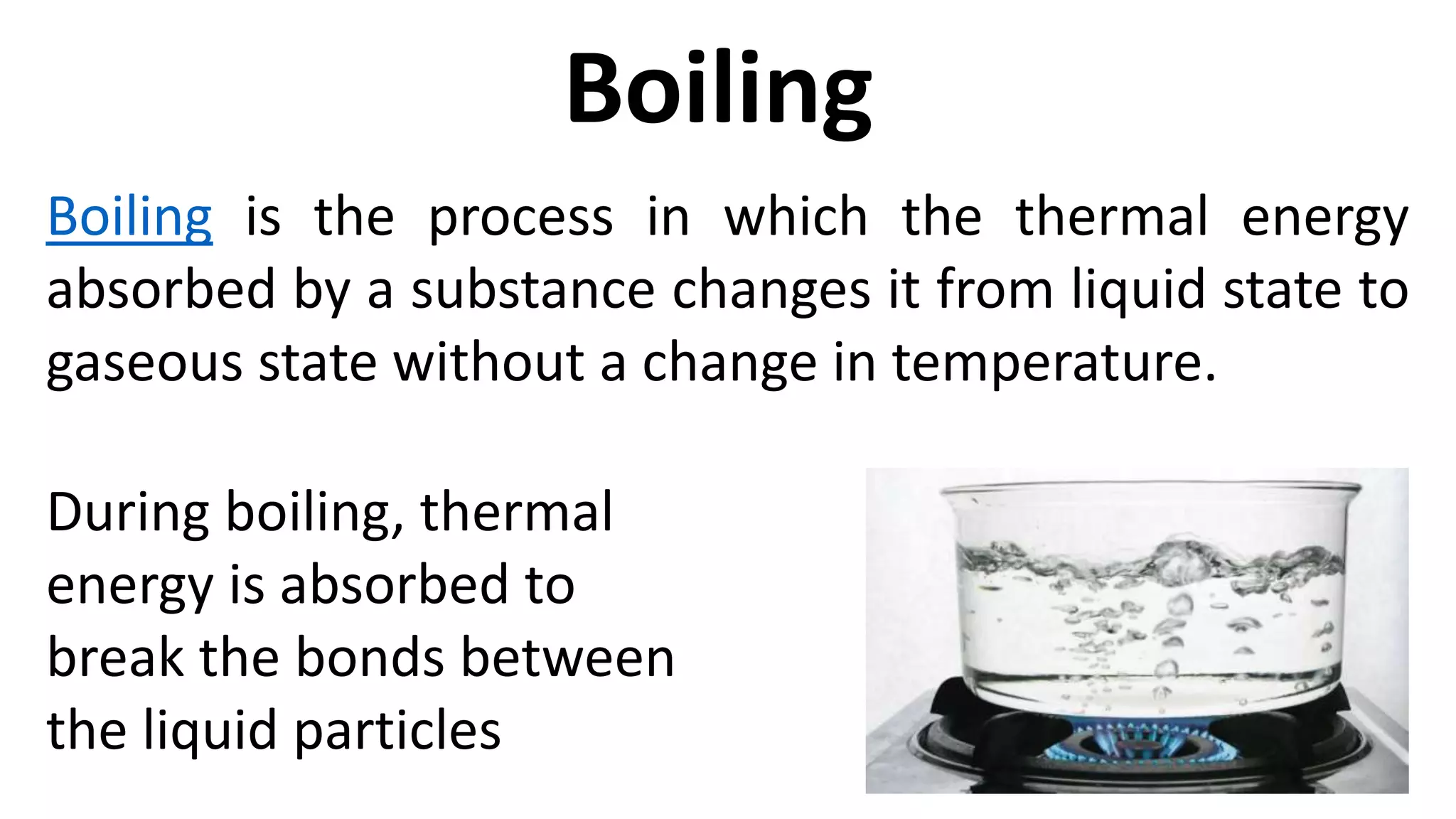
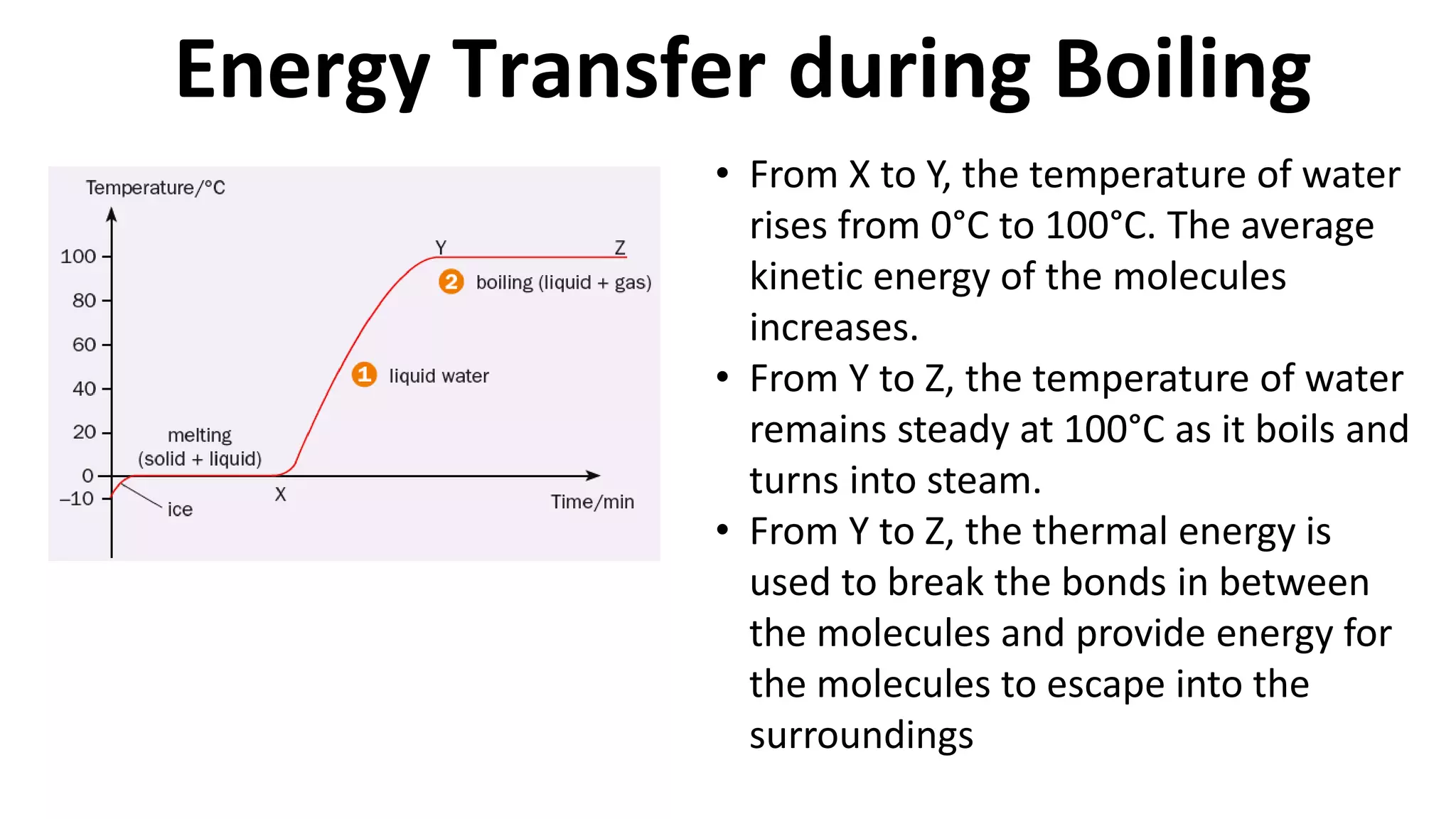
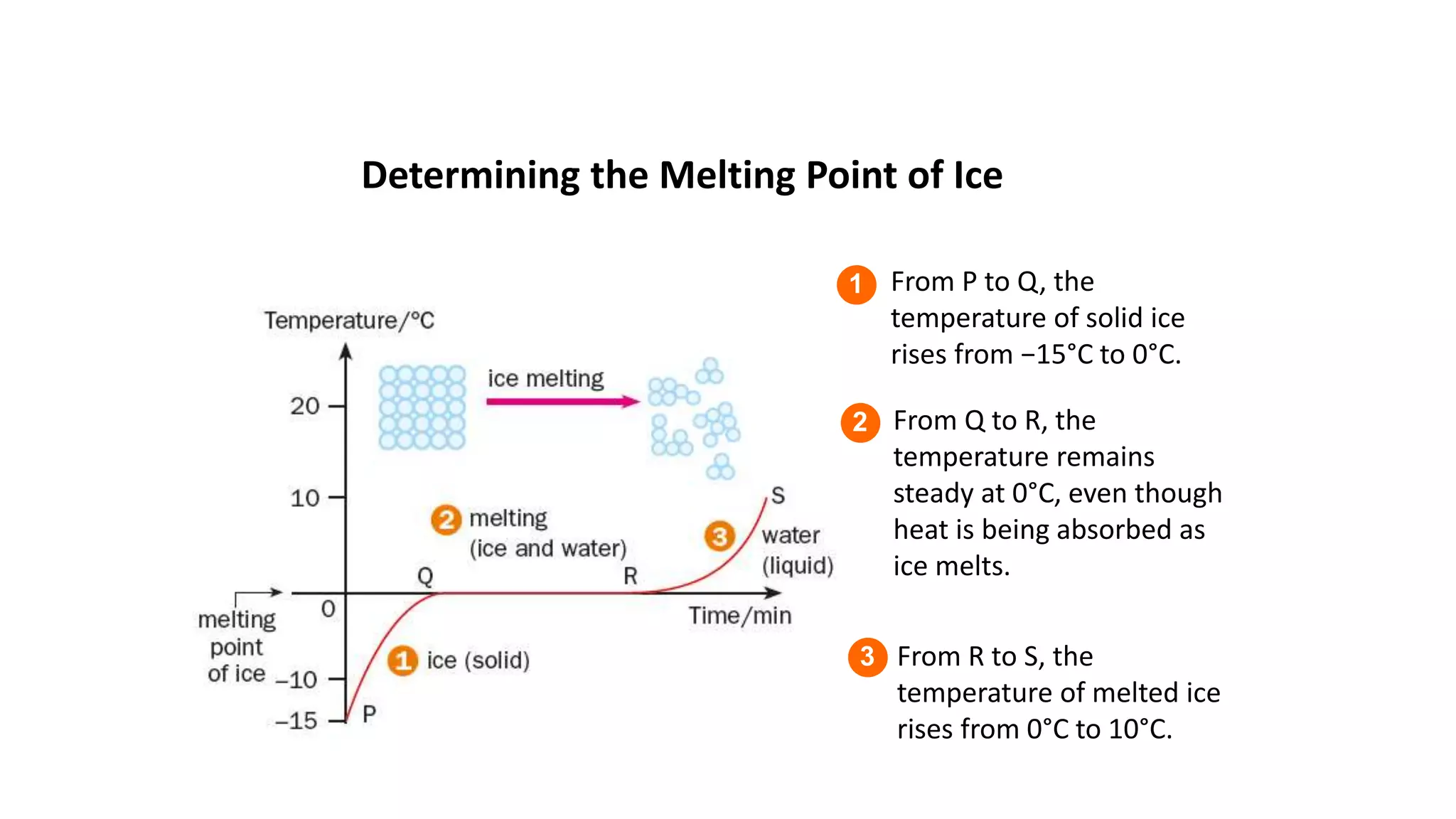
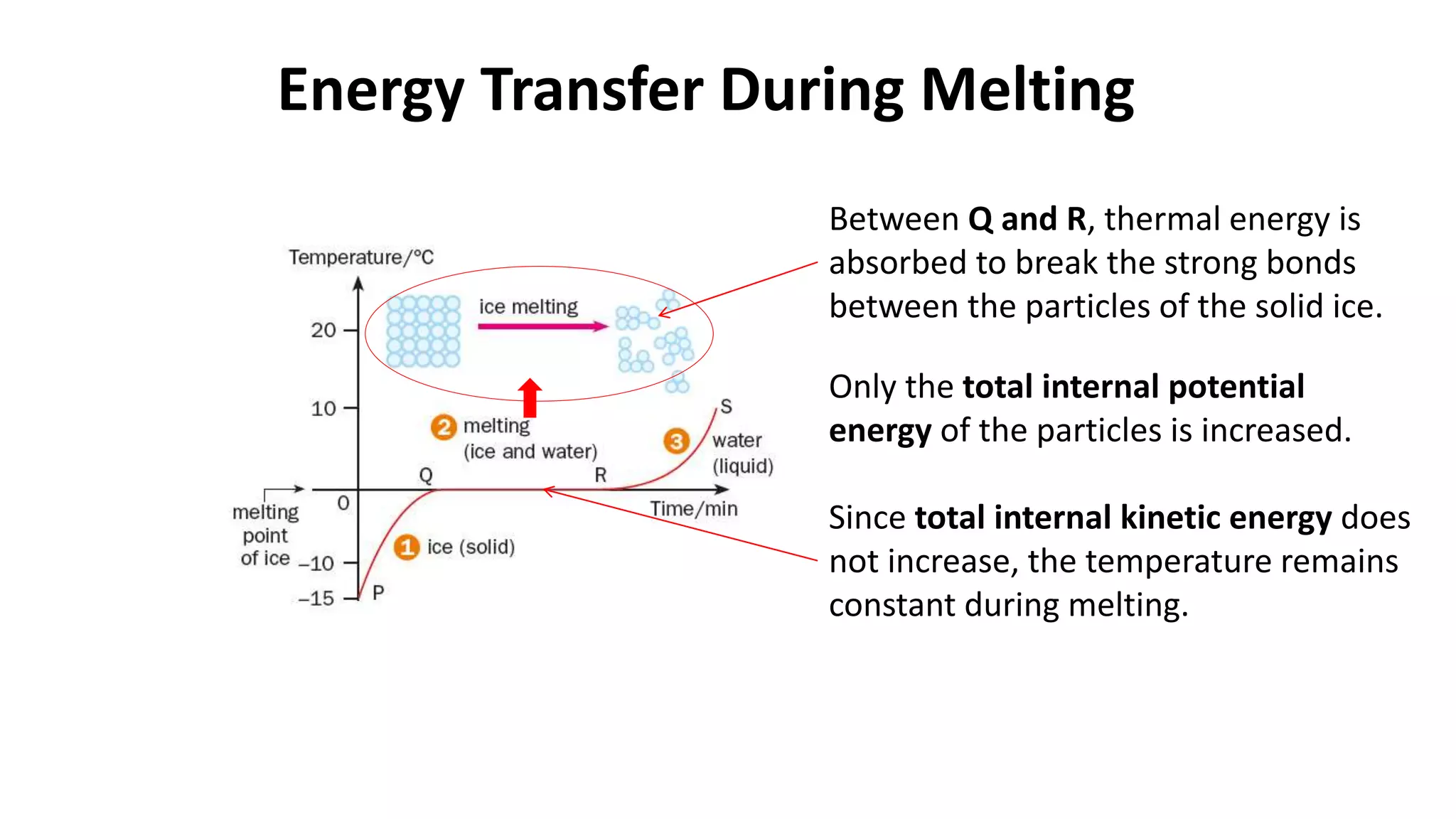
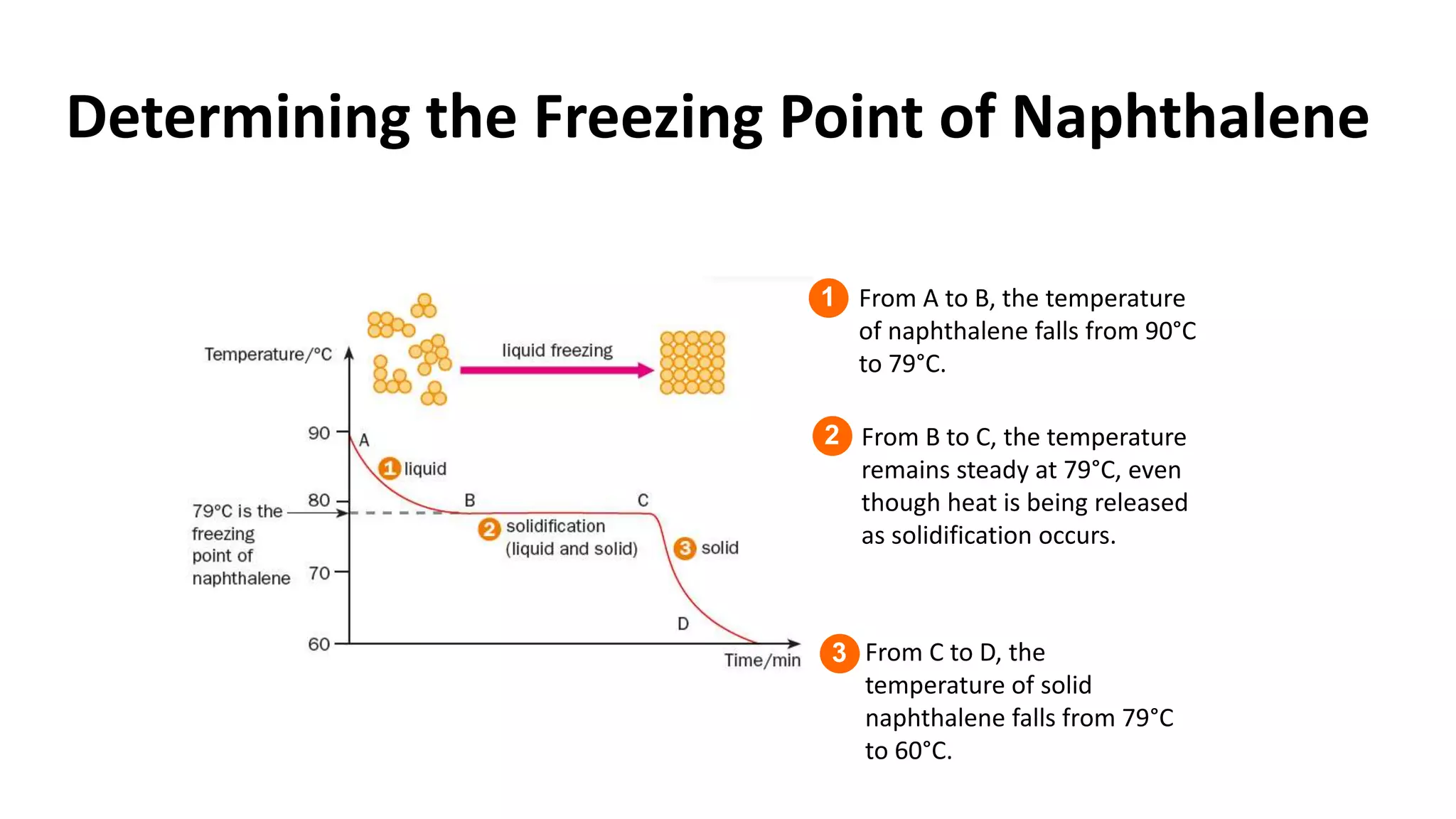
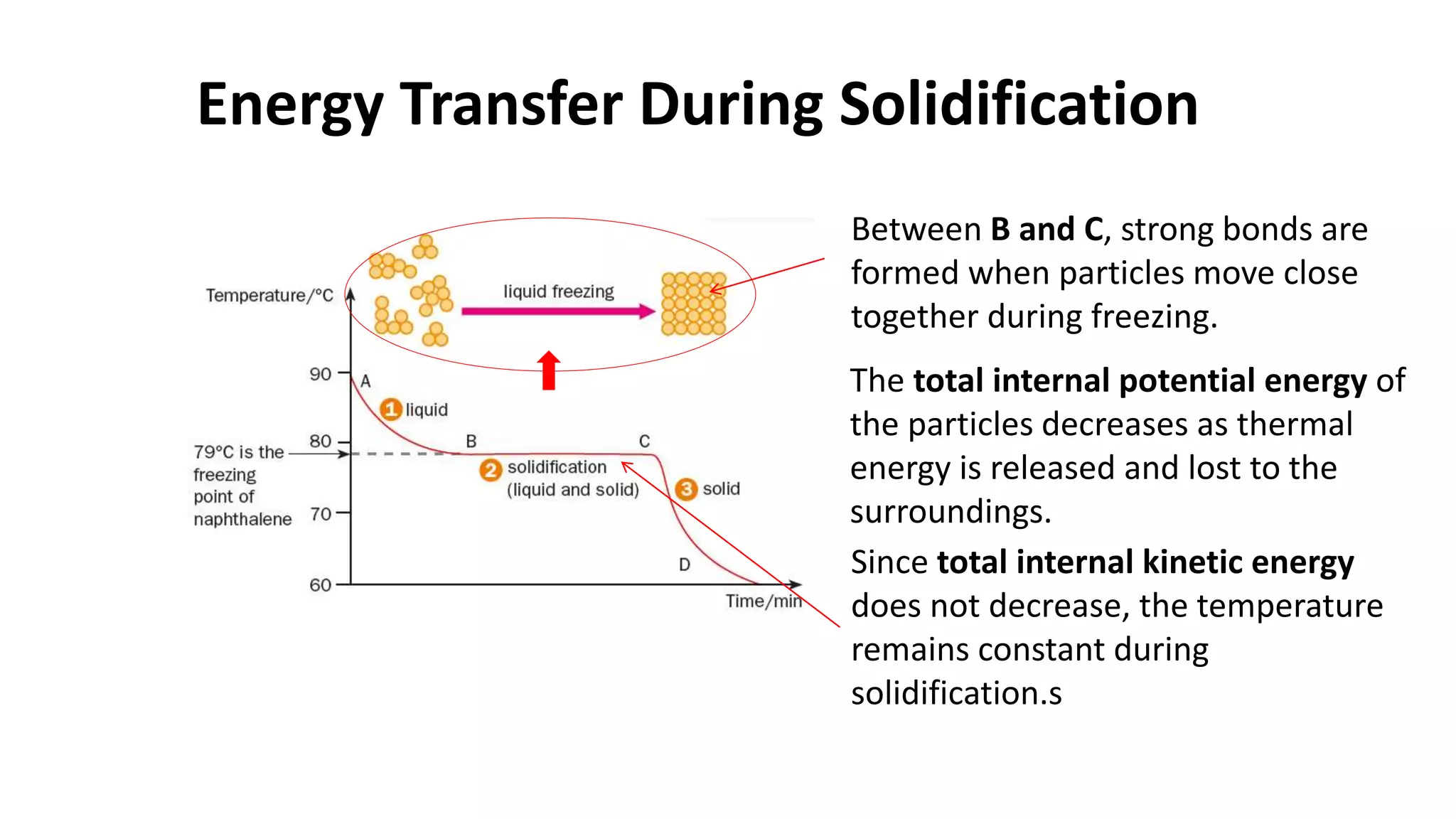
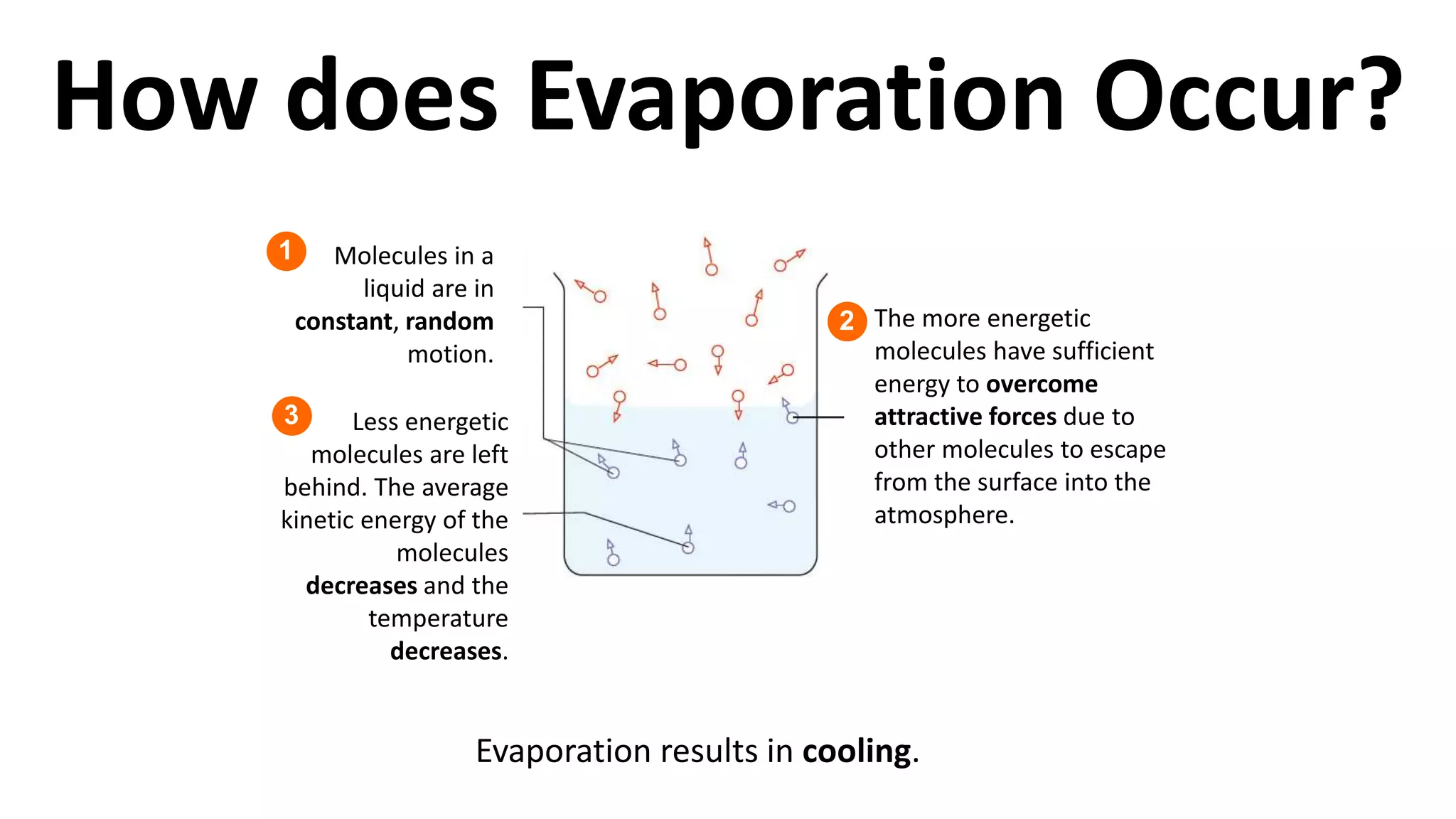
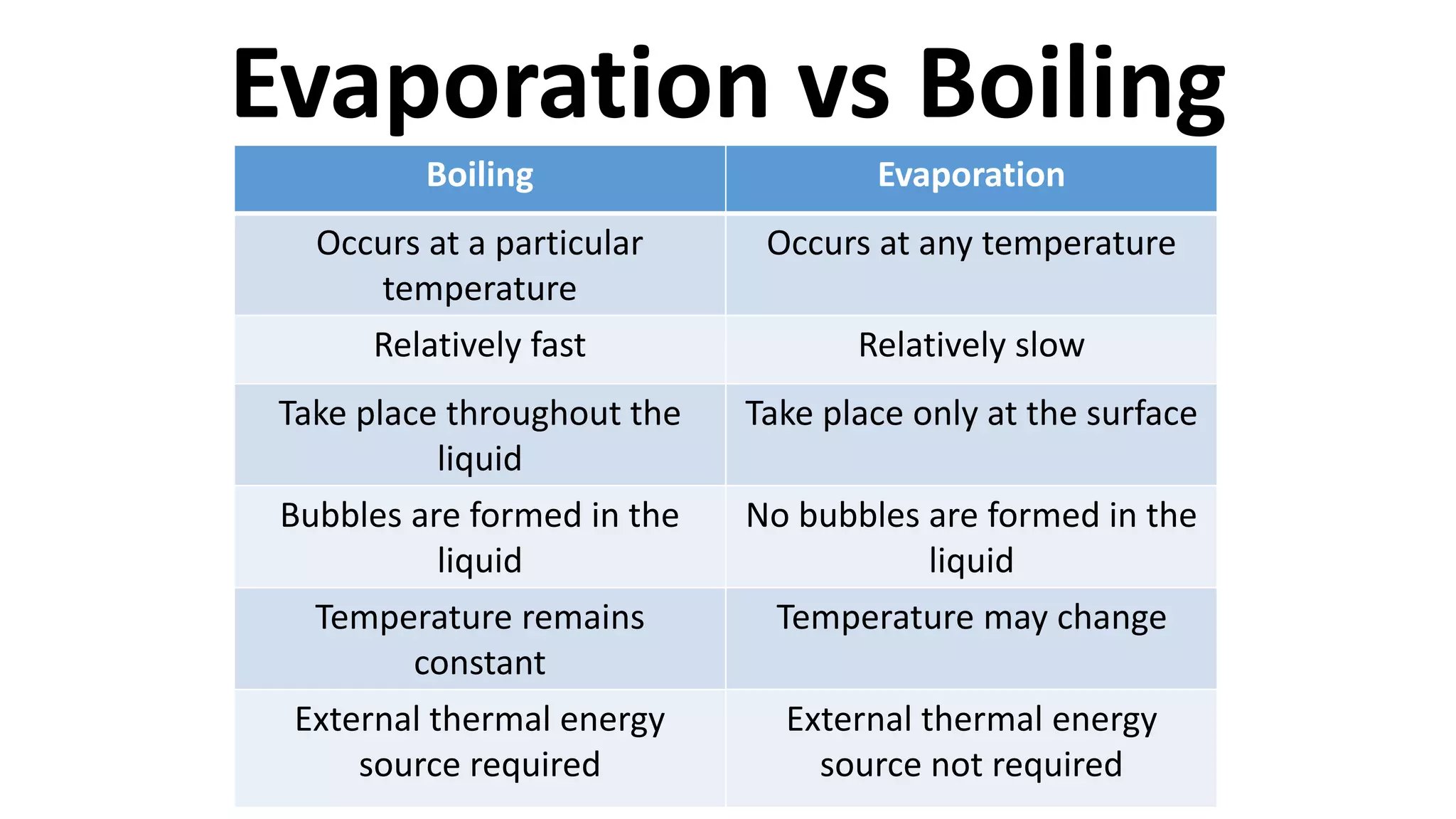
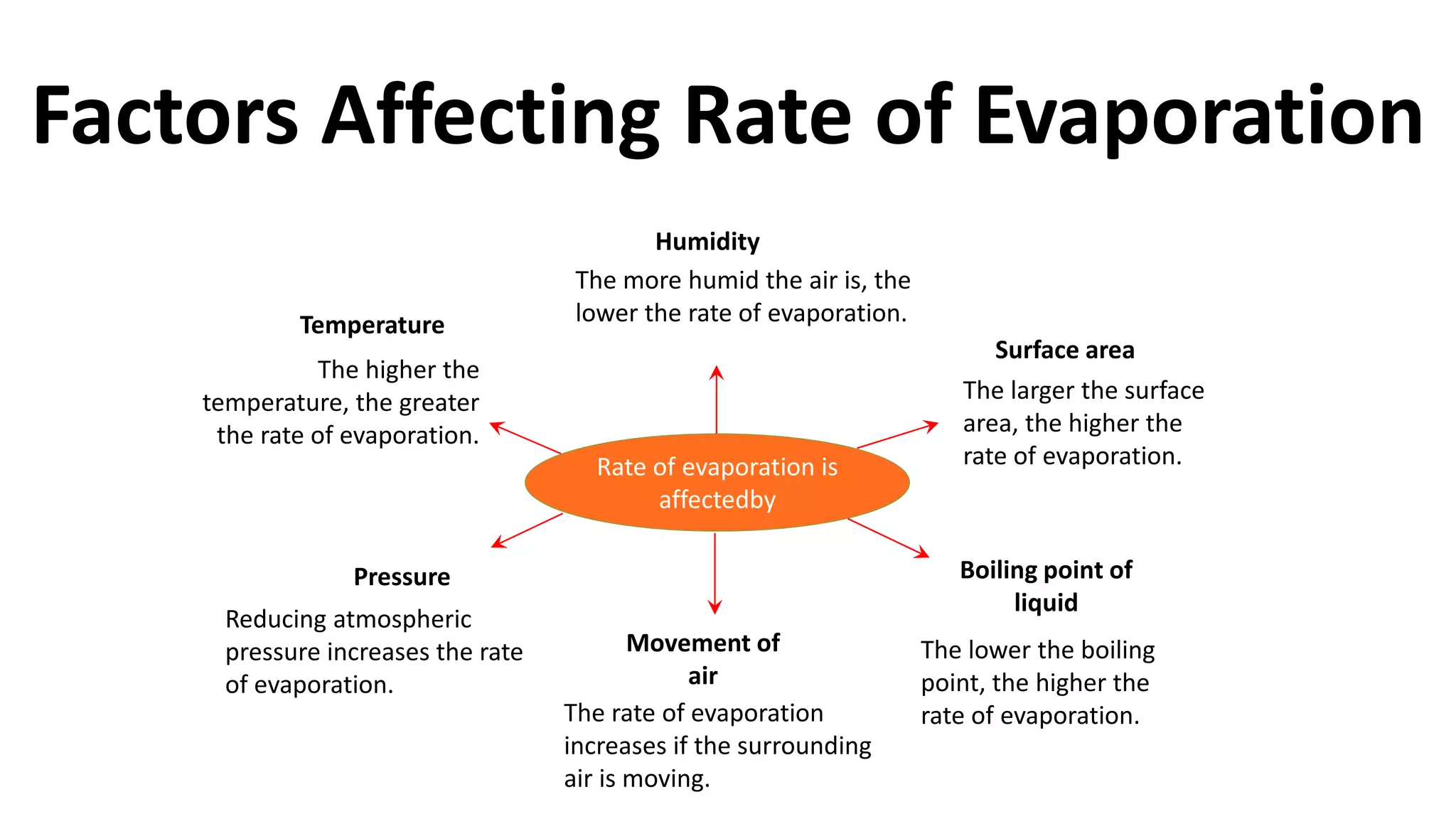
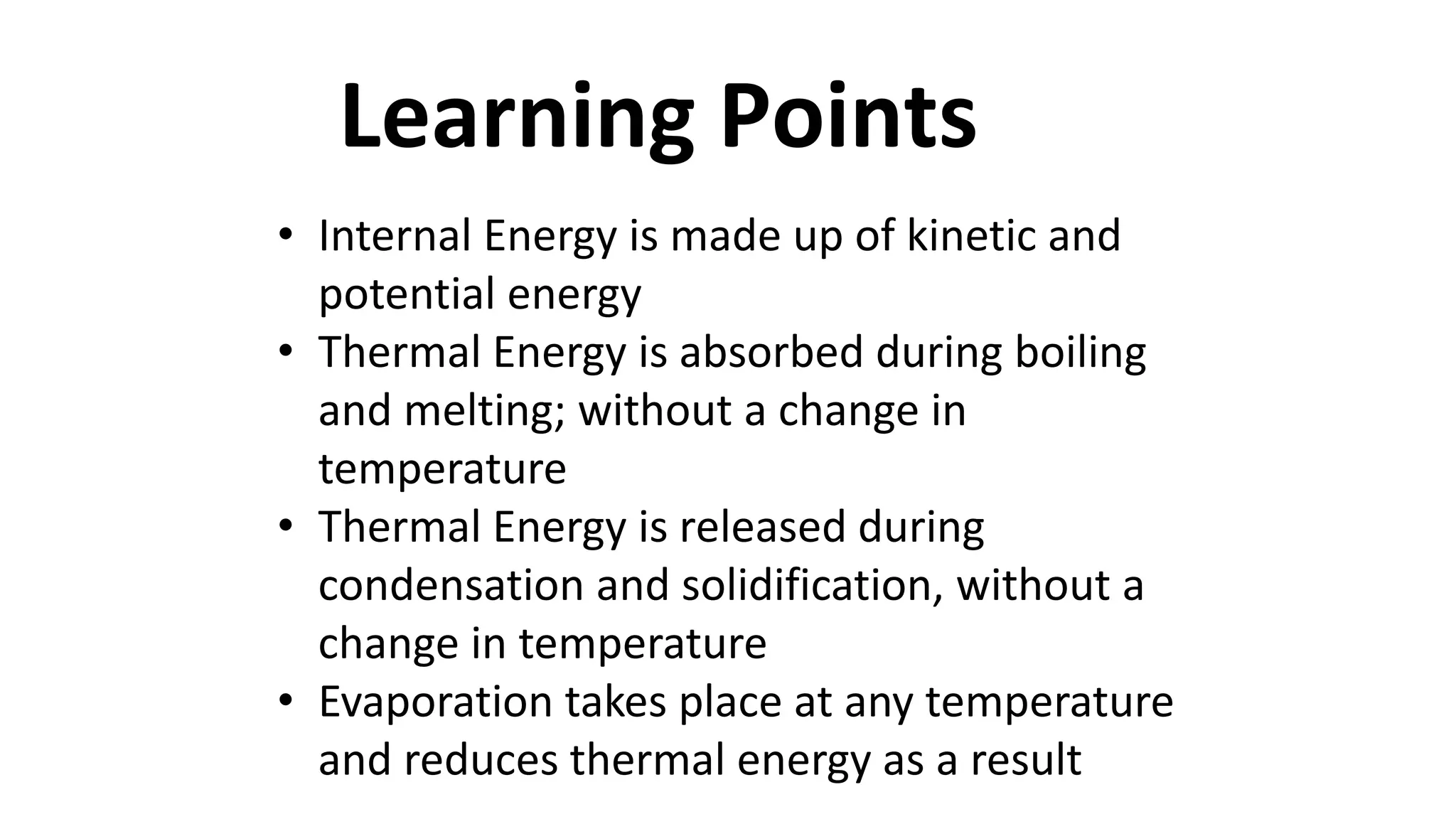
This document discusses different changes of state that matter undergoes. It explains that boiling and condensation involve a transfer of thermal energy without a change in temperature, where boiling transforms liquid to gas and condensation transforms gas to liquid. Melting and solidification also involve thermal energy transfer without temperature change, transforming between solid and liquid. Evaporation differs in that it can occur at any temperature and involves molecules at the surface of a liquid escaping into the air, lowering the liquid's temperature. Factors like temperature, pressure and surface area affect evaporation rates.