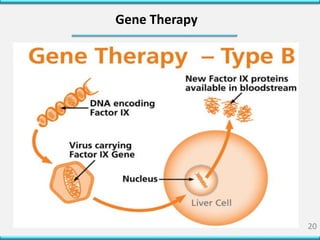
The document discusses hemophilia, a hereditary coagulation disorder characterized by deficiencies in clotting factors, mainly affecting males due to its X-linked recessive nature. It outlines the signs, symptoms, causes, diagnosis, and treatment options, including replacement therapy and emerging gene therapies. Additionally, it highlights the situation in Bangladesh, where proper diagnosis and treatment facilities for hemophilia are limited.