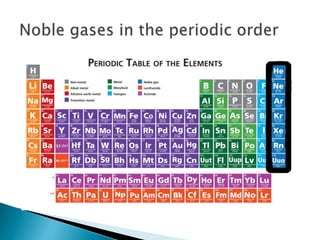
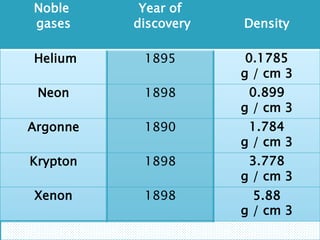
The document discusses the properties and uses of noble gases. It defines noble gases as elements that do not interact with other elements due to their complete electron orbits. It lists the noble gases as helium, neon, argon, krypton, xenon, and radon. It then discusses their physical properties, including being odorless and colorless gases at room temperature with low melting and boiling points. Finally, it provides examples of uses for some noble gases, such as using helium for balloons due to its light weight and neon for lighting due to its bright white color.