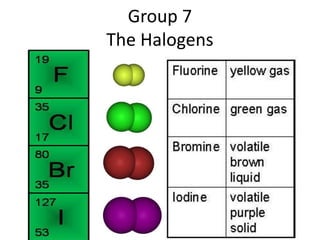
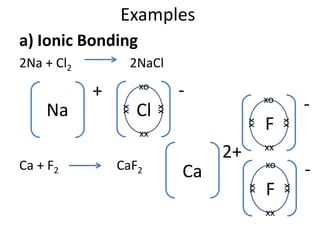
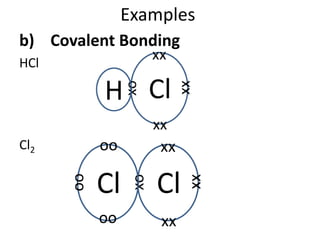
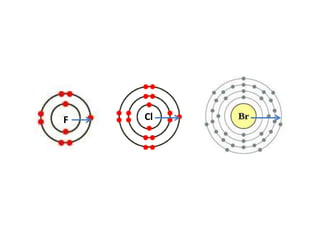
The document discusses the properties of group 7 elements (halogens) in the periodic table. It describes their:
1) Physical properties including their diatomic molecular structures, strong covalent bonds, low melting and boiling points, and poor conductivity.
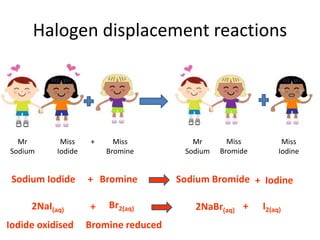
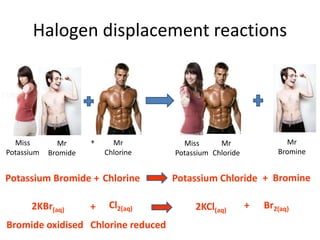
2) Chemical properties, specifically their tendency to gain one electron through ionic or covalent bonding reactions to achieve a full outer shell.
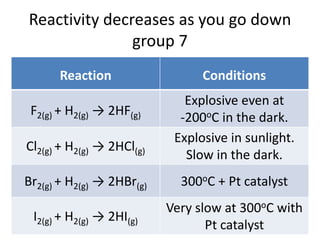
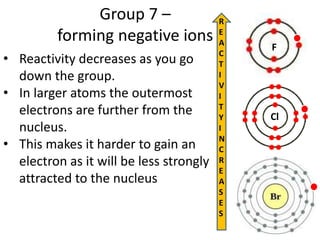
3) Reactivity decreases down the group as the outer electrons are farther from the nucleus, making ion formation harder. Fluorine is the most reactive halogen.