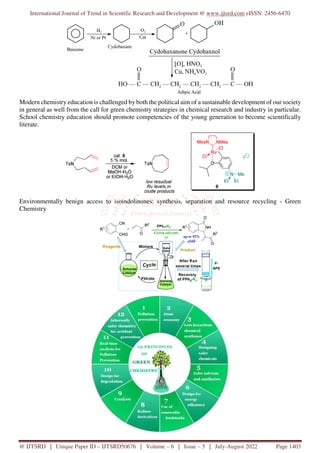
The document discusses the role of green chemistry in promoting sustainability within the chemical industry, emphasizing its importance for reducing waste, improving efficiency, and designing safer chemical processes. It outlines key principles of green chemistry that prioritize environmental and human health, while also addressing educational aspects that are crucial for preparing future chemists. Various examples and case studies illustrate the potential for green chemistry to create innovative and less hazardous materials, highlighting its significance in achieving broader societal sustainability goals.
![International Journal of Trend in Scientific Research and Development (IJTSRD)
Volume 6 Issue 5, July-August 2022 Available Online: www.ijtsrd.com e-ISSN: 2456 – 6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1402
Green Chemistry for a Sustainable Future
Dr. Mahesh Singh Khirwar
Associate Professor, Department of Chemistry, R.B.S. College, Agra, Uttar Pradesh, India
ABSTRACT
For more than three decades, Green Chemistry has provided a
framework for chemists and chemical engineers to do their part in
contributing to the broad scope of global sustainability. American
Chemical Society journals are a great venue for these scientists to
share their latest results and provide a resource to the chemistry
community and beyond for understanding current problems and
envisioning solutions. Sustainability in organic chemistry, especially
in organic synthesis, has been driving innovation for decades. With
the amount of waste generated in many synthetic chemistry routes,
especially at scale in manufacturing, we are faced with not only an
ethical imperative to develop more sustainable chemical processes
and products but also a financial imperative. Metrics to gauge our
progress, including process mass intensity (PMI), have been
developed that allow all aspects of a process to be compared. For
example, conducting a reaction in water may not necessarily be an
improvement if several volumes of an organic solvent are needed to
extract/purify the product. One could even argue that water can be
problematic because its high boiling point makes recycling energy
intensive, but we do not know these details without consciously
thinking about them. A recent perspective highlights the need to
explicitly include the assessment of sustainability using Green
Chemistry metrics.
KEYWORDS: green chemistry, sustainable, economy, waste, global,
manufacturing, metrics, recycling
How to cite this paper: Dr. Mahesh
Singh Khirwar "Green Chemistry for a
Sustainable Future" Published in
International Journal
of Trend in
Scientific Research
and Development
(ijtsrd), ISSN: 2456-
6470, Volume-6 |
Issue-5, August
2022, pp.1402-
1412, URL:
www.ijtsrd.com/papers/ijtsrd50676.pdf
Copyright © 2022 by author (s) and
International Journal of Trend in
Scientific Research and Development
Journal. This is an
Open Access article
distributed under the
terms of the Creative Commons
Attribution License (CC BY 4.0)
(http://creativecommons.org/licenses/by/4.0)
INTRODUCTION
Sustainable development is one of the most
frequently used terms in today's political debate. Our
current understanding of sustainable development as a
regulatory idea was basically defined by the Agenda
21: “Sustainable development is development that
meets the needs of the present without compromising
the ability of future generations to meet their own
needs.” As a consequence, all our individual and
political actions should be reflected in the light of
their societal, economical and ecological
sustainability. This claim concerns every field of
society, among them particularly chemistry and
chemistry education. Both fields should reflect on
how chemistry and chemistry education can
contribute to more sustainability in our society, today
and in future. One of chemistry's contributions to
meeting the challenge of more sustainability in the
development of our society is the promotion of a
sustainable chemistry, in research and industrial
production. Under the name of green chemistry (or in
Europe also sustainable chemistry) a lot of effort has
been undertaken to make future chemistry less
poisonous and less hazardous. Green chemistry aims
at making chemistry more energy efficient, at
reducing waste disposal, and/or producing innovative
products with less consumption of natural resources.
Alternative processes and reaction pathways are
designed, new materials and products are developed
contributing to meet our needs today, but with taking
more care of the interests of future generations.[1,2]
IJTSRD50676](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/75/Green-Chemistry-for-a-Sustainable-Future-1-2048.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1404
This means chemistry education has to contribute to making students capable of activelyparticipating in society.
Competencies need to be promoted to allow students to understand and participate in societal debate about
applications of chemistry and technology. One prerequisite is that students should achieve substantial chemistry
knowledge in the context of respective sustainability issues to understand the underlying developments,
alternatives and dilemmas. But, subject matter knowledge will not be enough. The students as future citizens
also need to learn how societal debate about questions related to chemistry, industry and the environment
functions as well as develop skills to involve themselves together with others in the societal processes of
democratic decision making.[3,4]
Potential points include:
Sustainable bio production of safer chemicals from food waste
Bioconversion of food waste into environmentally friendly reagents
Production, importation, utilization, development, adoption, and disposal of safer chemicals
Risk assessment and management associated with human health effects from chemical reagents
Green chemistry application to manage water engineering and water quality
Environmental chemistry approaches for pollution and accident prevention, safety and resource
sustainability, and energy and resource sustainability
Management of local, regional, and global impacts of air pollutants, including SOX, NOX, PM10/2.5, and
trace metals, with the design and implementation of safer chemicals, materials, and products
Environmental pollution and conservation, along with economic development, based on green chemistry
Regulatory aspects and hierarchy concepts of chemical waste management and pollution prevention
principles, utilizing knowledge of treatment, storage, and disposal facilities
State and federal agencies that govern the role of chemical technology in the industrialization of developed
and developing countries
Efficient use of natural resources through the utilization of renewable feed stocks
Green chemistry is the design of high-performing, cost-effective technology that is safer for the environment and
human health.[5,6]
“We’re in a world where it’s unequivocal that there are hazardous materials in commerce,” he says. “There are
carcinogens, endocrine disruptors, neurotoxins. There are materials that are causing global climate change and
ozone depletion. As a first step, we have to accept that these things are out there.” The next step, Warner attests,
is to assess how we got to this point in the first place. “Let’s assume that there are no monsters in the world,”
Warner says. “Let’s assume this really isn’t an epic battle of good and evil.” No scientist sets out to invent a red
pigment that causes cancer. No company prizes a plastic additive that can lead to birth defects over a safer
alternative. Why, then, do we continue to bring hazardous materials to market? The current state of regulation is
one problem (we’ll get to that later). But Warner says that the crux of the issue lies in what chemists are
taught—or rather not taught—in school. Warner arrived at this revelation through personal tragedy. In the 1990s,
he was a decade into his career as an industrial chemist. He had over 2,500 synthesized compounds to his credit,
when a rare birth defect led to the death of his two-year-old son. In grief, he was left to wonder about the
chemicals he’d created. Could any have contributed to his son’s illness? Not once in his many years in school,
including a PhD program at Princeton, did Warner take a course in toxicology. He hadn’t studied how molecules
persist in the environment. Today, nearly three decades later, such topics remain absent from core chemistry](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-3-320.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1405
curricula in the United States. “Pick up any organic chemistry textbook and compare it to one from 1980, [7,8]
and I challenge you to find a significant difference,” he says. You won’t even find references to the chemical
transformations that drive many industries today. “How in the world can we ask the inventors of technology to
make safe material if it’s not part of how they’ve learned to do chemistry?” Warner asks. “This is not an issue of
desire. It’s an issue of ability.”[9,10]
Important examples of green chemistry include: phasing out the use of chlorofluorocarbons (CFCs) in
refrigerants, which have played a role in creating the ozone hole; developing more efficient ways of making
pharmaceuticals, including the well-known painkiller ibuprofen and chemotherapy drug Taxol; and developing
cheaper, more efficient solar cells.
Making chemical compounds, particularlyorganic molecules (composed predominantly of carbon and hydrogen
atoms), is the basis of vast multinational industries from perfumes to plastics, farming to fabric, and dyes to
drugs. In a perfect world, these would be prepared from inexpensive, renewable sources in one practical,
efficient, safe and environmentally benign chemical reaction. Unfortunately, with the exception of the chemical
processes found in nature, the majority of chemical processes are not completely efficient, require multiple
reaction steps and generate hazardous by-products. [11,12]
While in the past traditional waste management strategies focused only on the disposal of toxic by-products,
today efforts have shifted to eliminating waste from the outset by making chemical reactions more efficient.
This adjustment has, in part, led to the advent of more sophisticated and effective catalytic reactions, which
reduce the amount of waste. The 2001 Chemistry Nobel Laureate Ryoji Noyori stressed that catalytic processes
represent “the only methods that offer the rational means of producing useful compounds in an economical,
energy-saving and environmentally benign way”.
Green chemistry will be one of the most important fields in the future. Although this field has developed rapidly
in the last 20 years, it is still at an early stage. Promoting green chemistry is a long-term task, and many](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-4-320.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1406
challenging scientific and technological issues need to be resolved; these are related to chemistry, material
science, engineering, environmental science, physics and biology. Scientists, engineers and industrialists should
work together to promote the development of this field. There is no doubt that the development and
implementation of green chemistry will contribute greatly to the sustainable development of our society.[13,14]
Discussion
Principles of Green Chemistry
1. Prevention: It is better to prevent waste than to treat or clean up waste after it has been created.
2. Atom Economy: Synthetic methods should be designed to maximize the incorporation of all materials used
in the process into the final product.
3. Less Hazardous: Chemical Syntheses Wherever practicable, synthetic methods should be designed to use and
generate substances that possess little or no toxicity to human health and the environment.
4. Designing Safer Chemicals: Chemical products should be designed to affect their desired function while
minimizing their toxicity.
5. Safer Solvents and Auxiliaries: The use of auxiliary substances (e.g., solvents, separation agents, etc.) should
be made unnecessary wherever possible and innocuous when used
6. Design for Energy Efficiency: Energy requirements of chemical processes should be recognized for their
environmental and economic impacts and should be minimized. If possible, synthetic methods should be
conducted at ambient temperature and pressure.
7. Use of Renewable Feedstock: A raw material or feedstock should be renewable rather than depleting
whenever technically and economically practicable.
8. Reduce Derivatives: Unnecessary derivatization (use of blocking groups, protection/deprotection, and
temporary modification of physical/chemical processes) should be minimized or avoided if possible, because
such steps require additional reagents and can generate waste.
9. Catalysis: Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
10. Design for Degradation: Chemical products should be designed so that at the end of their function they break
down into innocuous degradation products and do not persist in the environment.
11. Real-time analysis for Pollution Prevention: Analytical methodologies need to be further developed to allow
for real-time, in-process monitoring and control prior to the formation of hazardous substances.
12. Inherently Safer Chemistry for Accident Prevention: Substances and the form of a substance used in a
chemical process should be chosen to minimize the potential for chemical accidents, including releases,
explosions and fires.
Sustainable chemistry also embraces environmental chemistry, whereby fundamental chemical concepts such as
the p-block elements – C, N, O, P and S – are termed ‘nutrients’ and ‘salts’ are responsible for ‘salinity’ of soils
and surface waters. Pollutants disturb the natural nutrient cycles and salinity reduces soil and freshwater quality
with overall degradation of the natural environment. Similarly, increasing acidity of rivers and oceans disturbs
aquatic ecosystems and is a direct consequence of increased levels of carbon dioxide in the atmosphere.
Furthermore, increasing toxicity of the environment due to chemical waste in soils, air and surface waters is of
greatest concern in terms of addressing environmental sustainability.[15,16]](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-5-320.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1407
Sustainable chemistry intuitively involves engagement with the generation of new smart materials, and hence,
with nanotechnology and its envisaged linkages to global clean energy requirements. The rapidly advancing
nano-chemistry is perhaps the most significant exemplar of leading edge sustainable chemistry with its focus on
the development of new smart materials for energy storage, production and conversion, for advancing
agricultural productivity, water purification and desalination food processing, building construction, health
monitoring and for pest control. Of these applications, rapid advancement in the production of photo-voltaic
devices and carbon nano-tube solar cells is accelerating the solar energy industry. Similarly, the development of
nano-catalysts for hydrogen production, coupled with carbon nano-tube hydrogen storage systems are promoting
hydrogen as a viable, alternative clean energy resource. Thus, sustainable chemistry via nano-chemistry directly
engages with environmental sustainability by providing processes and products which directly benefit humanity
without harming the environment.[17,18]
Attempts are being made not only to quantify the greenness of a chemical process but also to factor in other
variables such as chemical yield, the price of reaction components, safety in handling chemicals, hardware](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-6-320.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1408
demands, energy profile and ease of product workup and purification. In one quantitative study, the reduction of
nitrobenzene to aniline receives 64 points out of 100 marking it as an acceptable synthesis overall whereas a
synthesis of an amide using HMDS is only described as adequate with a combined 32 points.
Green chemistry is increasingly seen as a powerful tool that researchers must use to evaluate the environmental
impact of nanotechnology. As nano materials are developed, the environmental and human health impacts of
both the products themselves and the processes to make them must be considered to ensure their long-term
economic viability. There is a trend of nano material technology in the practice, however, people ignored the
potential nanotoxicity. Therefore, people need to address further consideration on legal, ethical, safety, and
regulatory issues associated with nanomaterials [19]
In 1996, Dow Chemical won the 1996 Greener Reaction Conditions award for their 100% carbon dioxide
blowing agent for polystyrene foam production. Polystyrene foam is a common material used in packing and
food transportation. Seven hundred million pounds are produced each year in the United States alone.
Traditionally, CFC and other ozone-depleting chemicals were used in the production process of the foam sheets,
presenting a serious environmental hazard. Flammable, explosive, and, in some cases toxic hydrocarbons have
also been used as CFC replacements, but they present their own problems. Dow Chemical discovered that
supercritical carbon dioxide works equally as well as a blowing agent, without the need for hazardous
substances, allowing the polystyrene to be more easily recycled. The CO2 used in the process is reused from
other industries, so the net carbon released from the process is zero.
Addressing principle #2 is the Peroxide Process for producing hydrazine without cogenerating salt. Hydrazine is
traditionally produced by the Olin Raschig process from sodium hypochlorite (the active ingredient in many
bleaches) and ammonia. The net reaction produces one equivalent of sodium chloride for every equivalent of the
targeted product hydrazine:[27]
NaOCl + 2 NH3 → H2N-NH2 + NaCl + H2O
In the greener Peroxide process hydrogen peroxide is employed as the oxidant and the side product is water. The
net conversion follows:
2 NH3 + H2O2 → H2N-NH2 + 2 H2O
Addressing principle #4, this process does not require auxiliary extracting solvents. Methyl ethyl ketone is used
as a carrier for the hydrazine, the intermediate ketazine phase separates from the reaction mixture, facilitating
workup without the need of an extracting solvent.](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-7-320.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1409
In 2002, Cargill Dow (now Nature Works) won the Greener Reaction Conditions Award for their improved
method for polymerization of polylactic acid . Unfortunately, lactide-base polymers do not perform well and the
project was discontinued by Dow soon after the award. Lactic acid is produced by fermenting corn and
converted to lactide, the cyclic dimer ester of lactic acid using an efficient, tin-catalyzed cyclization. The L,L-
lactide enantiomer is isolated by distillation and polymerized in the melt to make a crystallizable polymer, which
has some applications including textiles and apparel, cutlery, and food packaging. Wal-Mart has announced that
it is using/will use PLA for its produce packaging. The Nature Works PLA process substitutes renewable
materials for petroleum feedstocks, doesn't require the use of hazardous organic solvents typical in other PLA
processes, and results in a high-quality polymer that is recyclable and compostable.[20]
In 2003 Shaw Industries selected a combination of
polyolefin resins as the base polymer of choice for
Eco Worx due to the low toxicity of its feed stocks,
superior adhesion properties, dimensional stability,
and its ability to be recycled. The EcoWorx
compound also had to be designed to be compatible
with nylon carpet fiber. Although EcoWorx may be
recovered from any fiber type, nylon-6 provides a
significant advantage. Polyolefins are compatible
with known nylon-6 depolymerization methods. PVC
interferes with those processes. Nylon-6 chemistry is
well-known and not addressed in first-generation
production. From its inception, EcoWorx met all of
the design criteria necessary to satisfy the needs of
the marketplace from a performance, health, and
environmental standpoint. Research indicated that
separation of the fiber and backing through
elutriation, grinding, and air separation proved to be
the best way to recover the face and backing
components, but an infrastructure for returning
postconsumer EcoWorx to the elutriation process was
necessary. Research also indicated that the
postconsumer carpet tile had a positive economic
value at the end of its useful life. EcoWorx is
recognized by MBDC as a certified cradle-to-cradle
design.
In 2005, Archer Daniels Midland (ADM) and
Novozymes won the Greener Synthetic Pathways
Award for their enzyme interesterification process. In
response to the U.S. Food and Drug Administration
(FDA) mandated labeling of trans-fats on nutritional
information by January 1, 2006, Novozymes and
ADM worked together to develop a clean, enzymatic
process for the interesterification of oils and fats by
interchanging saturated and unsaturated fatty acids.
The result is commercially viable products without
trans-fats. In addition to the human health benefits of
eliminating trans-fats, the process has reduced the use
of toxic chemicals and water, prevents vast amounts
of by products, and reduces the amount of fats and
oils wasted.
In 2011, the Outstanding Green Chemistry
Accomplishments by a Small Business Award went
to BioAmber Inc. for integrated production and
downstream applications of bio-based succinic acid.
Succinic acid is a platform chemical that is an
important starting material in the formulations of
everyday products. Traditionally, succinic acid is
produced from petroleum-based feedstocks.
BioAmber has developed process and technology that
produces succinic acid from the fermentation of
renewable feedstocks at a lower cost and lower
energy expenditure than the petroleum equivalent
while sequestering CO2 rather than emitting it.
However, lower prices of oil precipitated the
company into bankruptcy and bio-sourced succinic
acid is now barely made
Several laboratory chemicals are controversial from
the perspective of Green chemistry. The
Massachusetts Institute of Technology created a
"Green" Alternatives Wizard to help identify
alternatives. Ethidium bromide, xylene, mercury, and
formaldehyde have been identified as "worst
offenders" which have alternatives. Solvents in
particular make a large contribution to the
environmental impact of chemical manufacturing and
there is a growing focus on introducing Greener
solvents into the earliest stage of development of
these processes: laboratory-scale reaction and
purification methods. In the Pharmaceutical Industry,
both GSK and Pfizer have published Solvent
Selection Guides for their Drug Discovery
chemists.[19,20]
Results
In 2007, The EU put into place the Registration,
Evaluation, Authorisation and Restriction of
Chemicals (REACH) program, which requires](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-8-320.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1410
companies to provide data showing that their products
are safe. This regulation (1907/2006) ensures not only
the assessment of the chemicals' hazards as well as
risks during their uses but also includes measures for
banning or restricting/authorising uses of specific
substances. ECHA, the EU Chemicals Agency in
Helsinki, is implementing the regulation whereas the
enforcement lies with the EU member states. The
United States formed the Environmental Protection
Agency (EPA) in 1970 to protect human and
environmental health by creating and enforcing
environmental regulation. Green chemistry builds on
the EPA’s goals by encouraging chemists and
engineers to design chemicals, processes, and
products that avoid the creation of toxins and waste.
The U.S. law that governs the majority of industrial
chemicals (excluding pesticides, foods, and
pharmaceuticals) is the Toxic Substances Control Act
(TSCA) of 1976. Examining the role of regulatory
programs in shaping the development of green
chemistry in the United States, analysts have revealed
structural flaws and long-standing weaknesses in
TSCA; for example, a 2006 report to the California
Legislature concludes that TSCA has produced a
domestic chemicals market that discounts the
hazardous properties of chemicals relative to their
function, price, and performance. Scholars have
argued that such market conditions represent a key
barrier to the scientific, technical, and commercial
success of green chemistry in the U.S., and
fundamental policy changes are needed to correct
these weaknesses.
Passed in 1990, the Pollution Prevention Act helped
foster new approaches for dealing with pollution by
preventing environmental problems before they
happen.
Green chemistry grew in popularity in the United
States after the Pollution Prevention Act of 1990 was
passed. This Act declared that pollution should be
lowered by improving designs and products rather
than treatment and disposal. These regulations
encouraged chemists to reimagine pollution and
research ways to limit the toxins in the atmosphere. In
1991, the EPA Office of Pollution Prevention and
Toxics created a research grant program encouraging
the research and recreation of chemical products and
processes to limit the impact on the environment and
human health. The EPA hosts The Green Chemistry
Challenge each year to incentivize the economic and
environmental benefits of developing and utilizing
green chemistry.
In 2008, the State of California approved two laws
aiming to encourage green chemistry, launching the
California Green Chemistry Initiative. One of these
statutes required California's Department of Toxic
Substances Control (DTSC) to develop new
regulations to prioritize "chemicals of concern" and
promote the substitution of hazardous chemicals with
safer alternatives. The resulting regulations took
effect in 2013, initiating DTSC's Safer Consumer
Products Program.
There are ambiguities in the definition of green
chemistry, and in how it is understood among broader
science, policy, and business communities. Even
within chemistry, researchers have used the term
"green chemistry" to describe a range of work
independently of the framework put forward by
Anastas and Warner (i.e., the 12 principles).While not
all uses of the term are legitimate many are, and the
authoritative status of any single definition is
uncertain. More broadly, the idea of green chemistry
can easily be linked (or confused) with related
concepts like green engineering, environmental
design, or sustainability in general. The complexity
and multifaceted nature of green chemistry makes it
difficult to devise clear and simple metrics. As a
result, "what is green" is often open to debate. [18,19]
Green chemistry metrics describe aspects of a
chemical process relating to the principles of green
chemistry. The metrics serve to quantify the
efficiency or environmental performance of chemical
processes, and allow changes in performance to be
measured. The motivation for using metrics is the
expectation that quantifying technical and
environmental improvements can make the benefits
of new technologies more tangible, perceptible, or
understandable. This, in turn, is likely to aid the
communication of research and potentially facilitate
the wider adoption of green chemistrytechnologies in
industry.
For a non-chemist, an understandable method of
describing the improvement might be a decrease of X
unit cost per kilogram of compound Y. This,
however, might be an over-simplification. For
example, it would not allow a chemist to visualize the
improvement made or to understand changes in
material toxicity and process hazards. For yield
improvements and selectivity increases, simple
percentages are suitable, but this simplistic approach
may not always be appropriate. For example, when a
highly pyrophoric reagent is replaced by a benign
one, a numerical value is difficult to assign but the
improvement is obvious, if all other factors are
similar.
Numerous metrics have been formulated over time. A
general problem is that the more accurate and
universally applicable the metric devised, the more
complex and unemployable it becomes. A good](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-9-320.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1411
metric must be clearly defined, simple, measurable,
objective rather than subjective and must ultimately
drive the desired behavior.
Conclusions
Green chemistry is the design of chemical products
and processes that reduce and/or eliminate the use or
generation of hazardous substances. This approach
requires an open and interdisciplinary view of
material and product design, applying the principle
that it is better to consider waste and hazard
prevention options during the design and
development phase, rather than disposing, treating
and handling waste and hazardous chemicals after a
process or material has been developed. Green
chemistry is an opportunity for introducing innovative
solutions to chemical problems and applying
sustainability towards molecular design. Chemists
have the ability to design products and processes that
have reduced impacts on humans and the
environment and therefore creating sustainable
chemical building blocks for materials and products
in our society.[21]
References
[1] "Green Chemistry". United States
Environmental Protection Agency. 2006-06-
28. Retrieved 2011-03-23.
[2] Sheldon, R. A.; Arends, I. W. C. E.; Hanefeld,
U. (2007). Green Chemistry and Catalysis.
doi: 10.1002/9783527611003. ISBN
9783527611003. S2CID 92947071.
[3] Clark, J. H.; Luque, R.; Matharu, A. S.
(2012). "Green Chemistry, Biofuels, and
Biorefinery". Annual Review of Chemical and
Biomolecular Engineering. 3: 183–207. doi:
10.1146/annurev-chembioeng-062011-
081014. PMID 22468603.
[4] Cernansky, R. (2015). "Chemistry: Green
refill". Nature. 519 (7543): 379–380.
doi:10.1038/nj7543-379a. PMID 25793239.
[5] Sanderson, K. (2011). "Chemistry: It's not
easy being green". Nature. 469 (7328): 18–20.
Bibcode: 2011Natur. 469. . . 18S.
doi:10.1038/469018a. PMID 21209638.
[6] Poliakoff, M.; Licence, P. (2007).
"Sustainable technology: Green chemistry".
Nature. 450 (7171): 810–812. Bibcode:
2007Natur. 450. . 810P. doi:10.1038/450810a.
PMID 18064000. S2CID 12340643.
[7] Clark, J. H. (1999). "Green chemistry:
Challenges and opportunities". Green
Chemistry. 1: 1–8. doi: 10. 1039/A807961G.
[8] Marteel, Anne E.; Davies, Julian A.; Olson,
Walter W.; Abraham, Martin A. (2003).
"GREEN CHEMISTRY AND
ENGINEERING: Drivers, Metrics, and
Reduction to Practice". Annual Review of
Environment and Resources. 28: 401–428.
doi:10.1146/annurev.energy.28.011503.
163459.
[9] Vert, Michel; Doi, Yoshiharu; Hellwich, Karl-
Heinz; Hess, Michael; Hodge, Philip; Kubisa,
Przemyslaw; Rinaudo, Marguerite; Schué,
François (2012). "Terminology for biorelated
polymers and applications (IUPAC
Recommendations 2012)" (PDF). Pure and
Applied Chemistry. 84 (2): 377–410.
doi:10.1351/PAC-REC-10-12-
04.S2CID98107080.
[10] Woodhouse, E. J.; Breyman, S. (2005).
"Green chemistry as social movement?".
Science, Technology, & Human Values. 30
(2): 199–222.
doi:10.1177/0162243904271726.S2CID14677
4456.
[11] Linthorst, J. A. (2009). "An overview: Origins
and development of green chemistry".
Foundations of Chemistry. 12: 55–68. doi:
10.1007/s10698-009-9079-4.
[12] Anastas, Paul T.; Warner, John C. (1998).
Green chemistry: theory and practice. Oxford
[England]; New York: Oxford University
Press. ISBN 9780198502340.
[13] "12 Principles of Green Chemistry - American
Chemical Society". American Chemical
Society. Retrieved 2018-02-16.
[14] Van Aken, K.; Strekowski, L.; Patiny, L.
(2006). "EcoScale, a semi-quantitative tool to
select an organic preparation based on
economical and ecological parameters".
Beilstein Journal of Organic Chemistry. 2 (1):
3. doi: 10.1186/1860-5397-2-3. PMC
1409775. PMID 16542013.
[15] "Green nanotechnology" (PDF). Archived
from the original (PDF) on 2016-04-06.
Retrieved 2008-03-01.
[16] Nanotoxicology toxicity evaluation of
nanomedicine applications. Hemant Kumar
Daima, Shanker Lal Kothari, Bhargava
Suresh Kumar. Boca Raton. 2021. ISBN 978-
1-000-39991-2. OCLC 1256699945.](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-10-320.jpg)
![International Journal of Trend in Scientific Research and Development @ www.ijtsrd.com eISSN: 2456-6470
@ IJTSRD | Unique Paper ID – IJTSRD50676 | Volume – 6 | Issue – 5 | July-August 2022 Page 1412
[17] Torok, Bela (2017). Green Chemistry: An
Inclusive Approach. Amsterdam: Elsevier. p.
Ch 3. 15.
[18] Prat, D.; Pardigon, O.; Flemming, H. -W.;
Letestu, S.; Ducandas, V.; Isnard, P.;
Guntrum, E.; Senac, T.; Ruisseau, S.;
Cruciani, P.; Hosek, P. (2013). "Sanofi's
Solvent Selection Guide: A Step Toward
More Sustainable Processes". Org. Process
Res. Dev. 17 (12): 1517–1525. doi:
10.1021/op4002565.
[19] Sherman, J.; Chin, B.; Huibers, P. D. T.;
Garcia-Valls, R.; Hatton, T. A. (1998).
"Solvent Replacement for Green Processing".
Environ. Health Perspect. 106 (Suppl 1): 253–
271. doi: 10.2307/3433925. JSTOR 3433925.
PMC 1533296. PMID 9539018.
[20] Isoni, V. (2016). "Q-SAOESS: A
methodology to help solvent selection for
pharmaceutical manufacture at the early
process development stage". Green Chem. 18:
6564. doi: 10.1039/C6GC02440H.
[21] Clarke, Coby J.; Tu, Wei-Chien; Levers,
Oliver; Brohl, Andreas; Hallett, Jason P.
(2018). "Green and Sustainable Solvents in
Chemical Processes". Chemical Reviews. 118
(2): 747–800.
doi:10.1021/acs.chemrev.7b00571.
hdl:10044/1/59694. PMID 29300087.](https://image.slidesharecdn.com/182greenchemistryforasustainablefuture-220906111428-f776ff69/85/Green-Chemistry-for-a-Sustainable-Future-11-320.jpg)