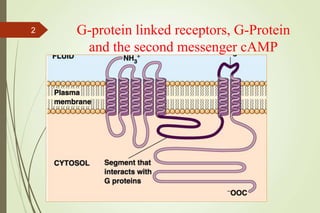
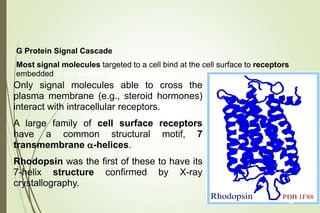
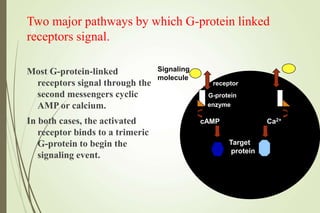
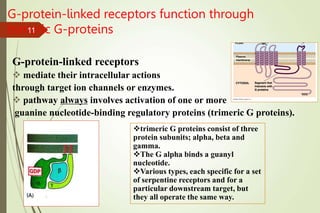
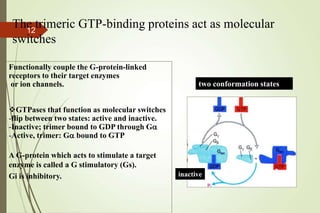
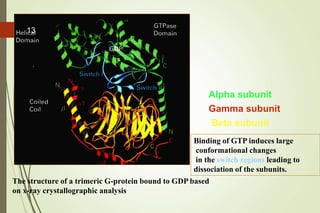
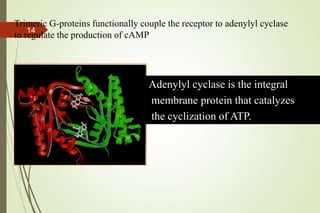
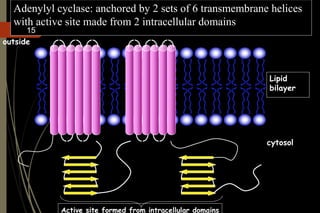
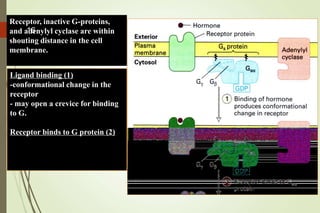
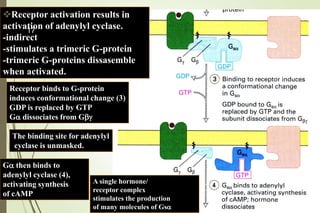
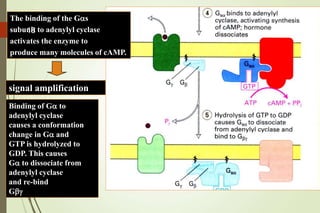
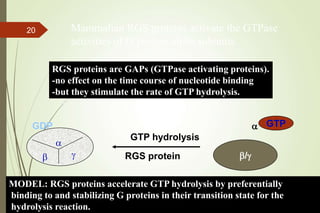
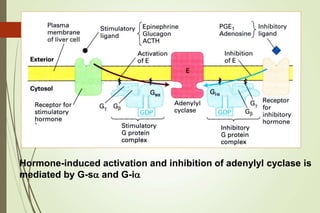
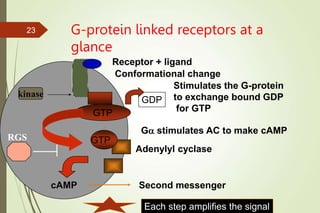
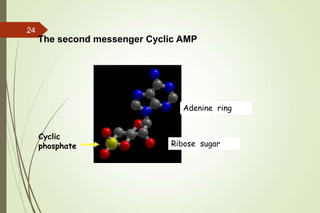
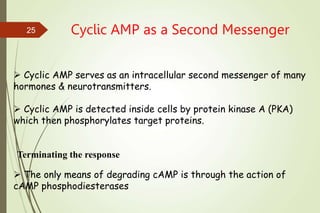
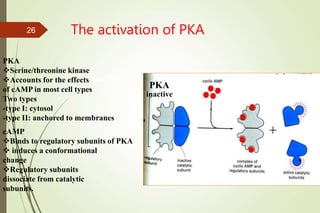
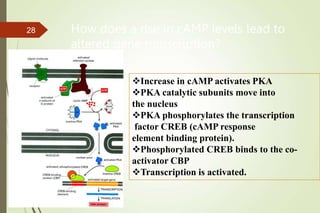
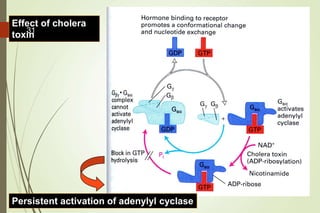
G-protein coupled receptors (GPCRs) are the largest family of cell surface receptors and mediate cellular responses to a wide range of signaling molecules. They signal through trimeric G-proteins, which functionally couple the receptors to target enzymes like adenylyl cyclase. Some G-proteins activate adenylyl cyclase to produce the second messenger cAMP, while others inhibit it. cAMP functions through protein kinase A to regulate metabolism and gene transcription. Cholera toxin causes prolonged cAMP production by permanently activating G-proteins, leading to severe diarrhea.