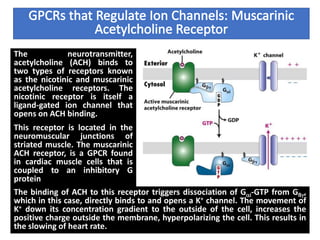
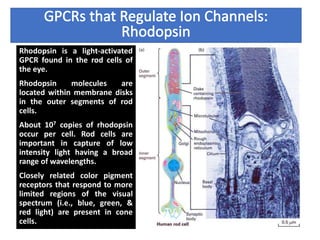
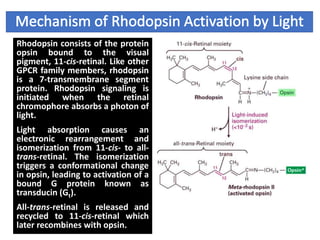
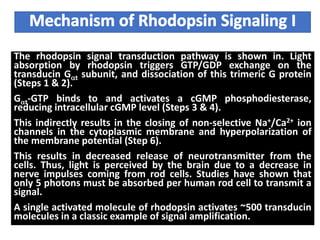
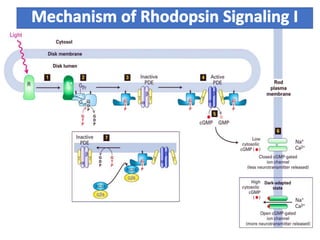
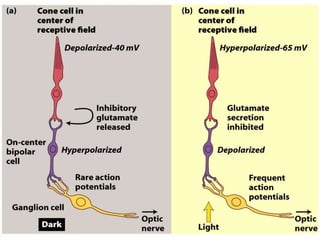
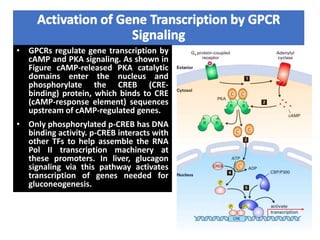
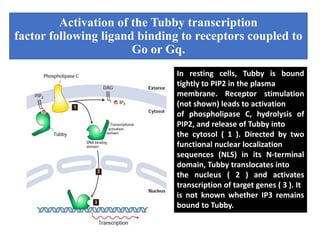
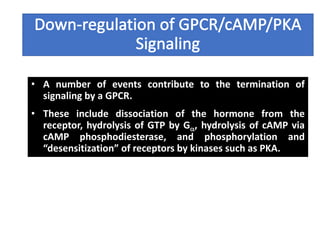
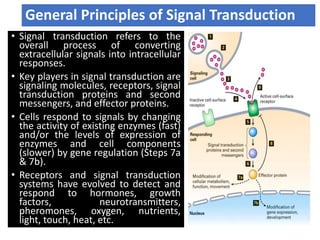
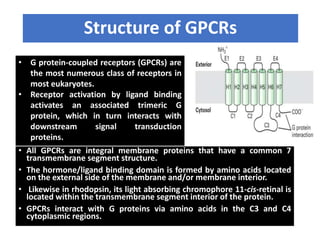
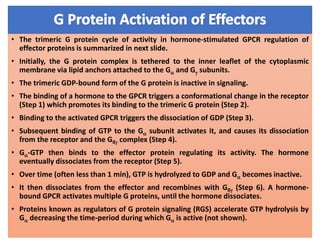
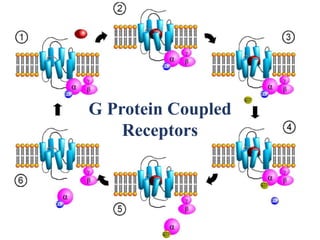
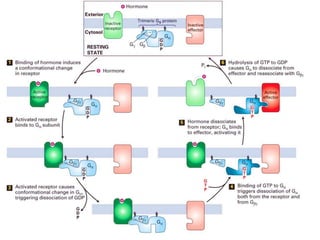
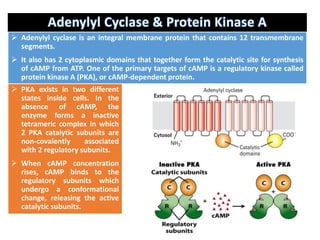
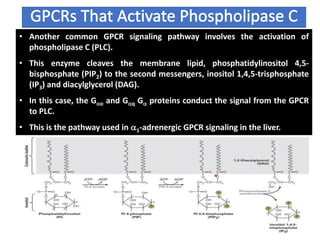
The document discusses the general principles of signal transduction, focusing on G protein-coupled receptors (GPCRs) and their mechanisms in cellular responses. It describes the structure of GPCRs, their interaction with G proteins, and how they regulate various cellular functions including the synthesis of cAMP and the activation of phospholipase C. Additionally, it covers the role of GPCR signaling in gene transcription and emphasizes the significance of signal amplification in these pathways.




![The steps downstream of PLC that make up the IP3/DAG signaling pathway are
illustrated in given picture.
IP3 diffuses from the cytoplasmic membrane to the ER where it binds to and triggers the
opening of IP3-gated Ca2+ channels (Steps 3 & 4).
Another kinase, protein kinase C (PKC) binds to DAG in the cytoplasmic membrane and is
activated (Step 6).
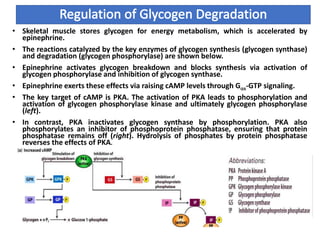
In liver, the rise in cytoplasmic [Ca2+] activates enzymes such as glycogen phosphorylase
kinase, which phosphorylates and activates glycogen phosphorylase. Glycogen
phosphorylase kinase is activated by Ca2+-calmodulin. In addition, PKC phosphorylates
and inactivates glycogen synthase.](https://image.slidesharecdn.com/gpcrsignalling-171201181902/85/Gpcr-signalling-13-320.jpg)
![• A related signaling pathway involving phospholipase C operates in vascular
endothelial cells and causes adjacent smooth muscle cells to relax in
response to circulating acetylcholine.
• In the NO/cGMP signaling pathway, the downstream target of
Ca2+/calmodulin is nitric oxide synthase, which synthesizes the gas NO from
arginine. NO diffuses into smooth muscle cells and causes relaxation by
activating guanylyl cyclase and increasing [cGMP].
• As a result arteries in tissues such as the heart dilate, increasing blood supply
to the tissue. NO also is produced from the drug nitroglycerin which is given
to heart attack patients and patients being treated for angina.](https://image.slidesharecdn.com/gpcrsignalling-171201181902/85/Gpcr-signalling-14-320.jpg)