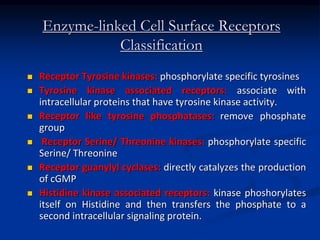
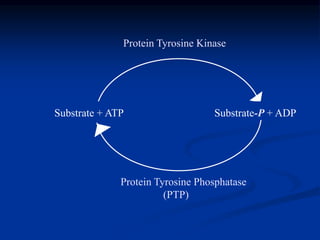
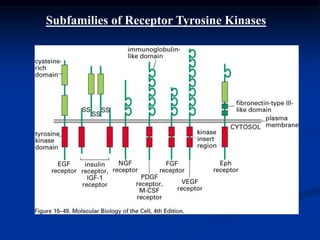
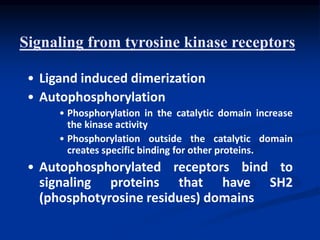
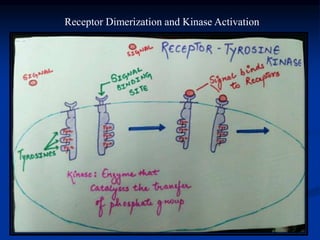
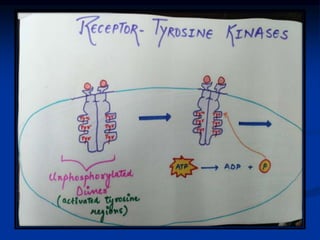
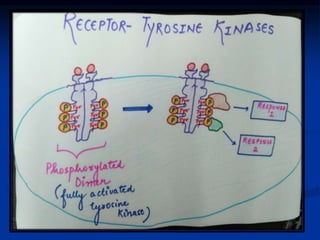
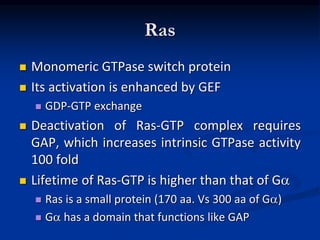
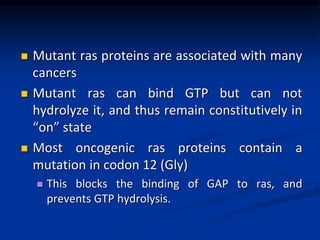
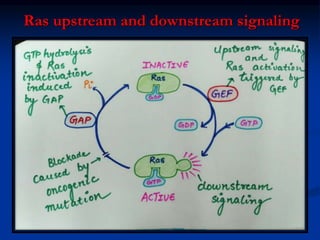
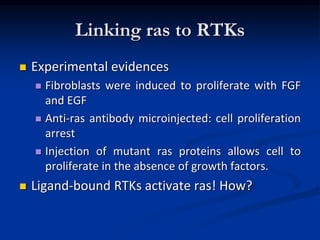
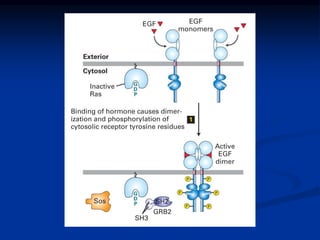
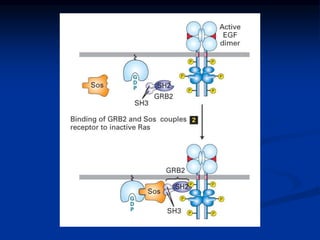
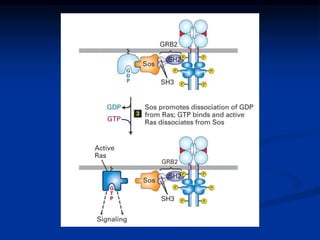
This document discusses enzyme-linked cell surface receptors, specifically receptor tyrosine kinases (RTKs). It classifies RTKs and notes they intrinsically possess tyrosine kinase activity. Upon ligand binding, RTKs dimerize and autophosphorylate, activating downstream signaling pathways like Ras-MAPK. RTK activation leads to cell proliferation, survival, and metabolism. Mutations in RTKs like HER2 and EGFR are implicated in some cancers. The document also outlines RTK signaling, including how binding partners GRB2 and Sos link RTK activation to Ras activation, transmitting the signal inside the cell.