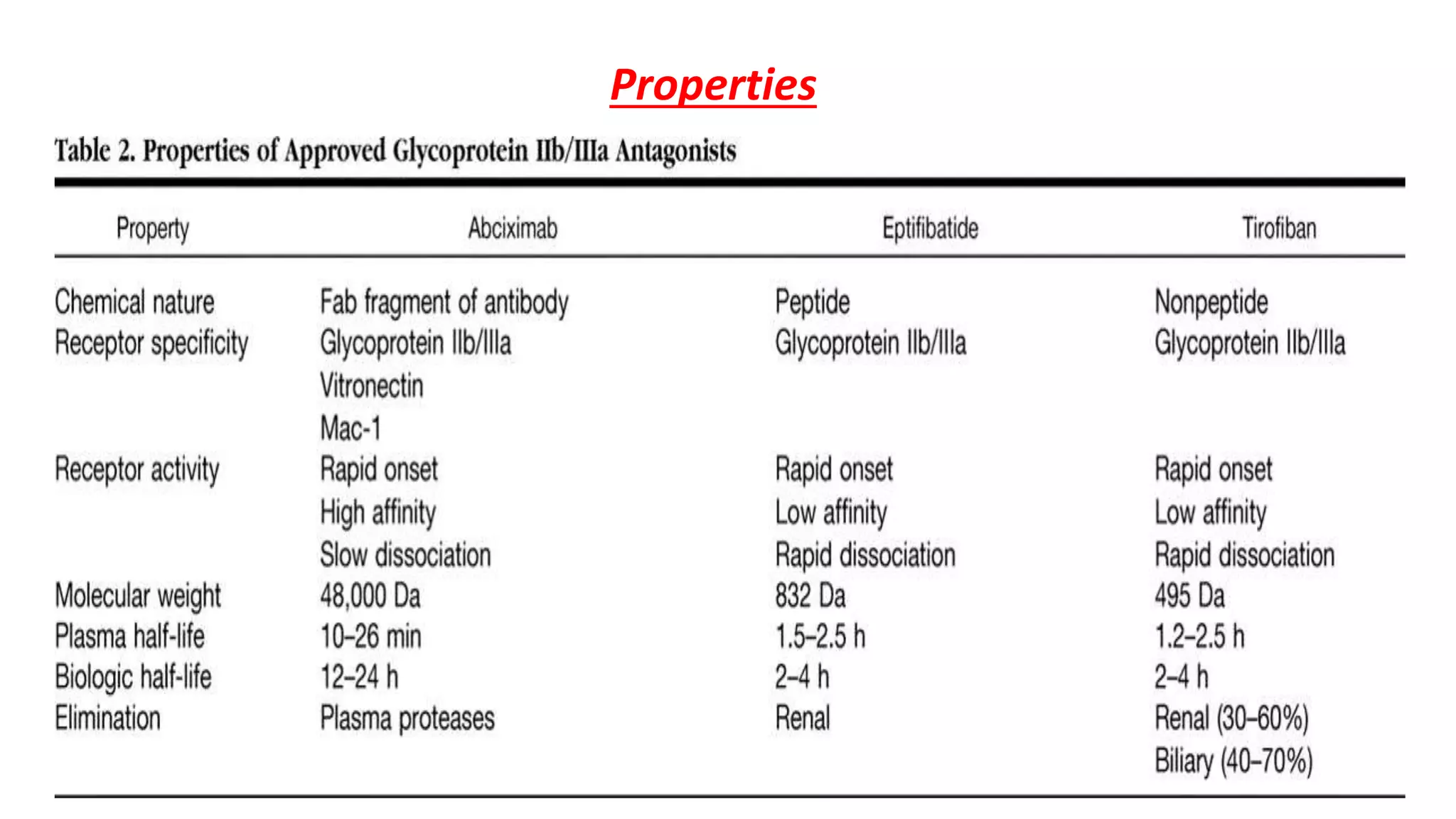
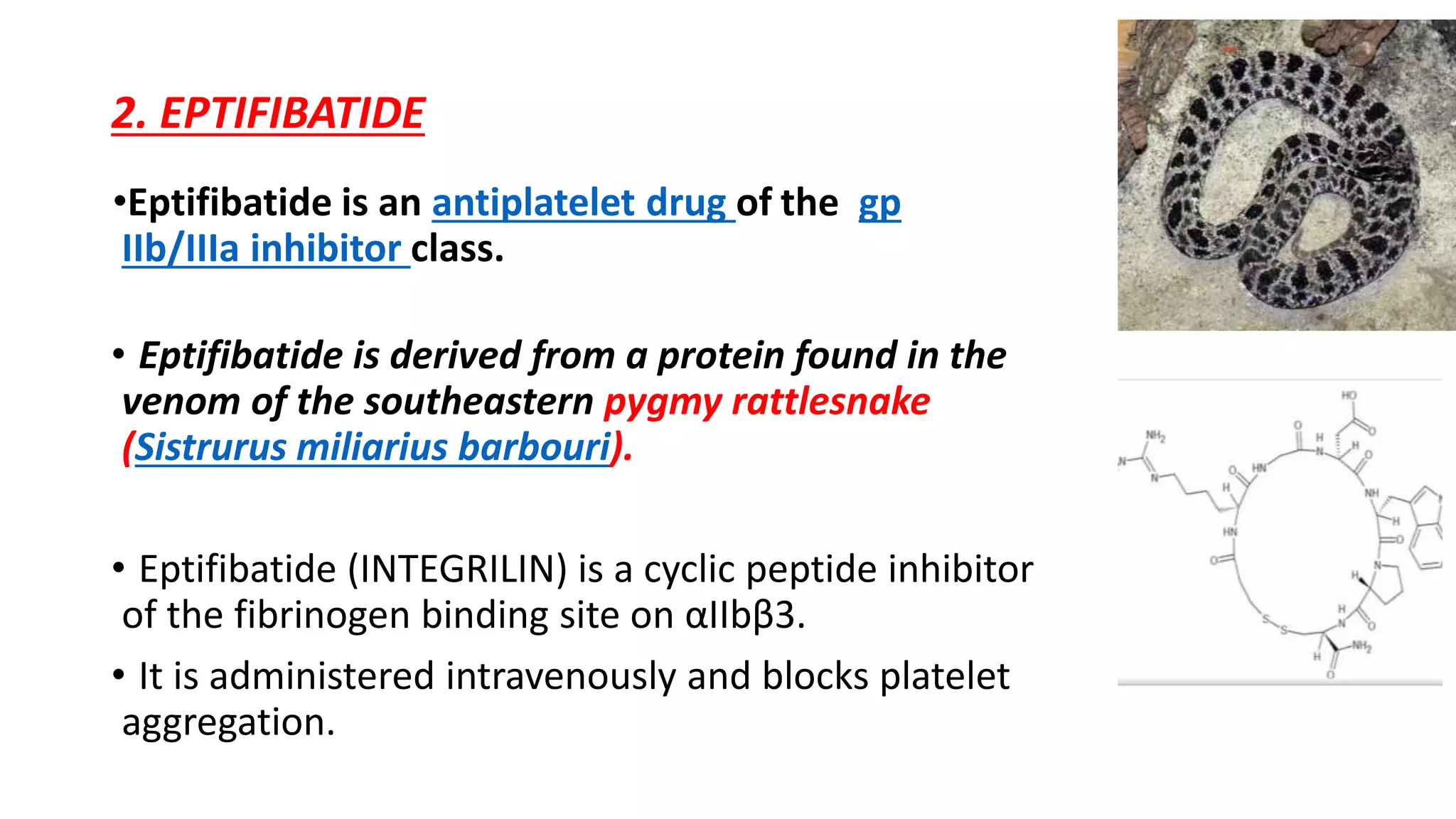
GP IIb/IIIa inhibitors are a class of drugs that block the GP IIb/IIIa receptor on platelets to prevent aggregation. The three main GP IIb/IIIa inhibitors are abciximab, eptifibatide, and tirofiban. They are used during percutaneous coronary interventions and for acute coronary syndromes to reduce complications by inhibiting the final common pathway of platelet aggregation. While generally well-tolerated, bleeding is a common side effect due to their antiplatelet effects.