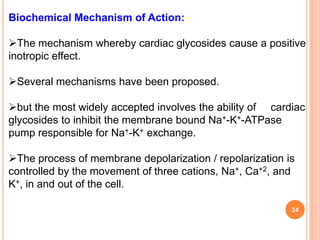
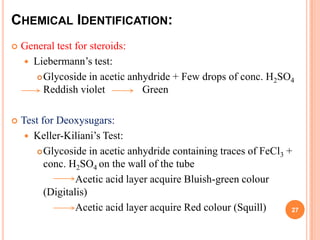
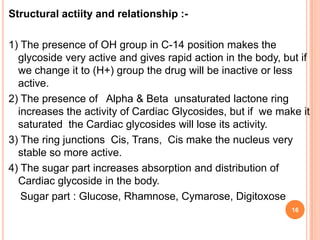
1) Cardiac glycosides are plant compounds that have beneficial and toxic effects on the heart. They work by inhibiting sodium-potassium pumps in cardiac muscle cells.
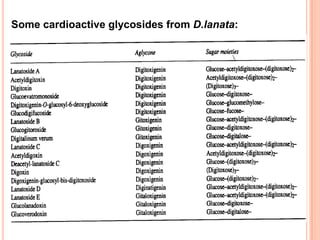
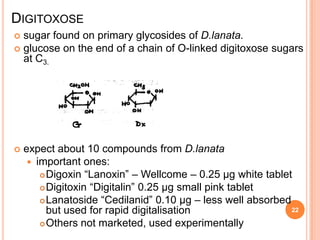
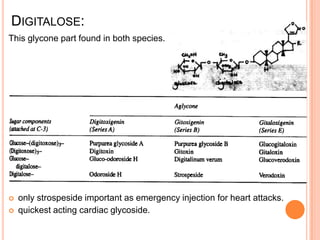
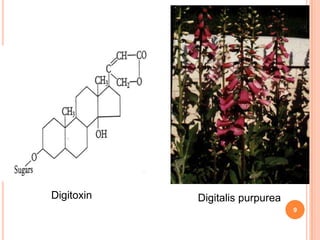
2) Key sources of cardiac glycosides include foxglove (Digitalis purpurea), squill bulbs, and seeds from Strophanthus plants. These sources contain glycosides like digoxin, digitoxin, and ouabain.
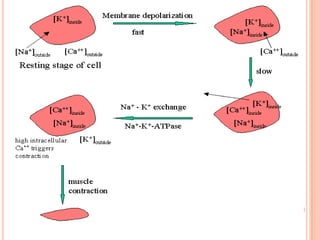
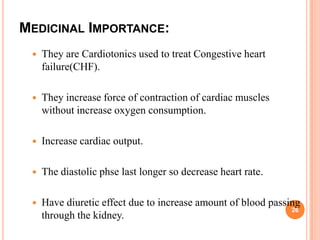
3) Cardiac glycosides increase the force of cardiac muscle contractions and the strength of the heart's pumping action, making them useful for treating congestive heart failure.








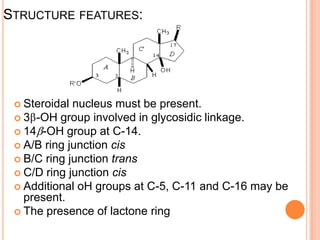



![CHEMISTRY OF D.LANATA
compounds belong to
cardenolide series
5 membered lactone ring
approx 96 compounds
[1930-1950 Stroll
worked on structures]
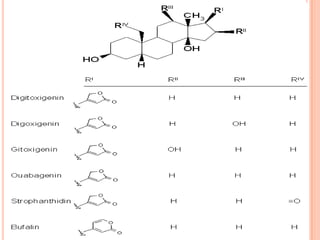
R1 R2 Names 1y 2y
H H digitoxigenin A A digitoxin
H OH gitoxigenin B B gitoxin
OH H digoxigenin C C digoxin
OH OH diginatigenin D D diginatin
H formylester gitaloxigenin E E gitaloxin
*
* Acetyl group
confers crystalline
properties - makes
compounds more
easily isolated](https://image.slidesharecdn.com/cardiacglycosides-140417070902-phpapp01/85/Cardiac-glycosides-20-320.jpg)