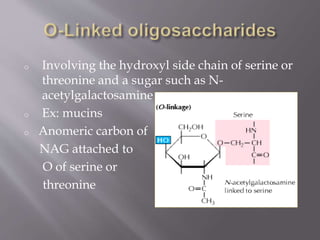
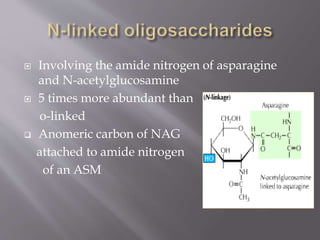
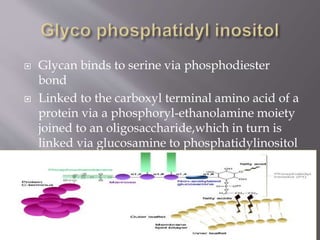
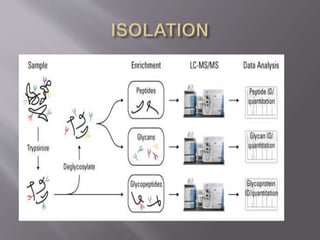
Glycoproteins are proteins that have covalently bound carbohydrates. They can be classified into three groups: O-linked oligosaccharides, N-linked oligosaccharides, and glyco phosphatidyl inositol. Glycoproteins serve various functions like being structural molecules, lubricants, regulating enzymes and transport molecules, and affecting protein folding. Glycoproteins can be purified using lectins in affinity chromatography and detected using periodic acid-Schiff stain. Studies found that glycoproteins degrade more rapidly than unfractionated proteins and that liver glycoproteins tend to be larger and more acidic than average proteins.