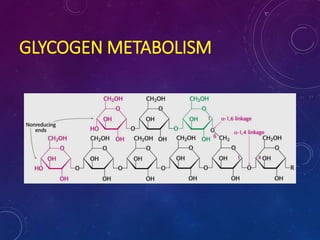
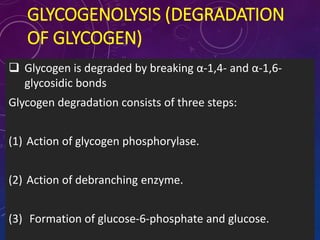
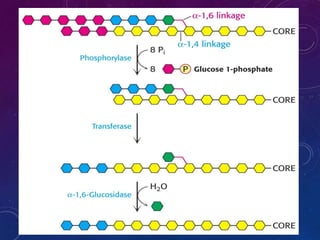
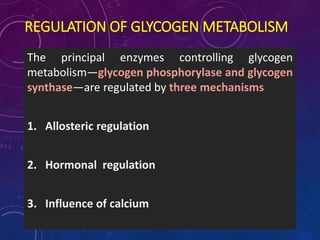
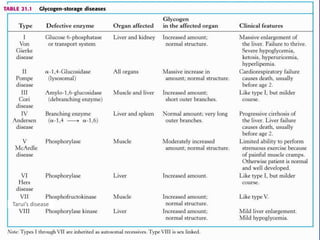
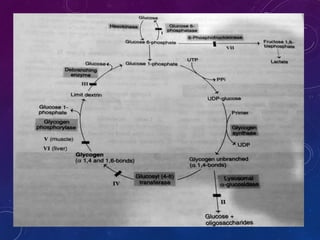
Glycogen is a key energy storage form of glucose, primarily stored in the liver and muscles, where it serves to maintain blood glucose levels and provide energy during physical activity. The synthesis (glycogenesis) and breakdown (glycogenolysis) of glycogen involve various enzymatic processes and are tightly regulated by hormones and cellular energy status. Glycogen storage diseases represent inherited disorders affecting glycogen metabolism, resulting in abnormal glycogen accumulation or mobilization.