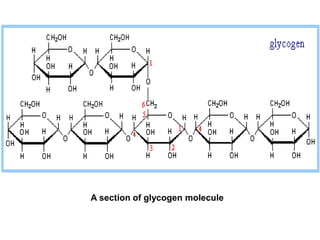
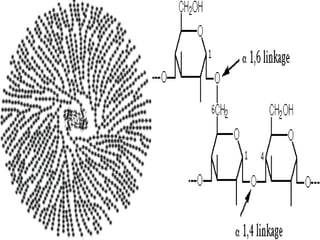
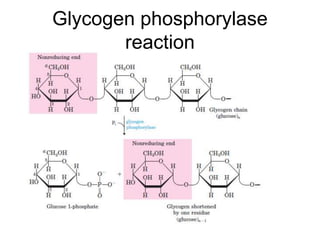
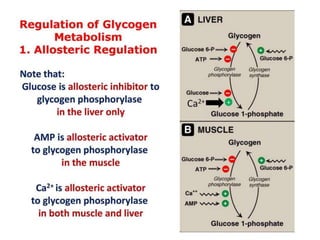
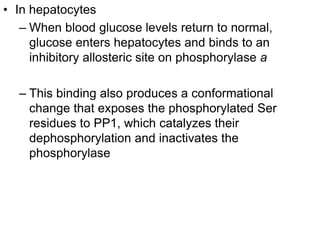
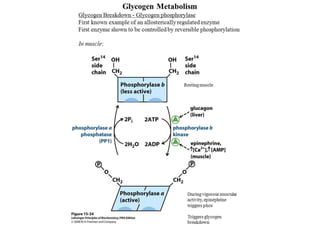
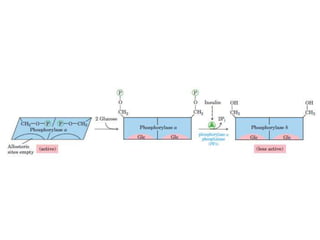
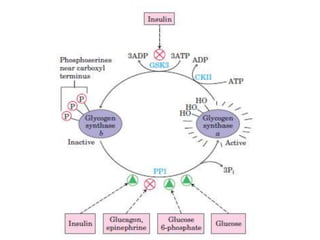
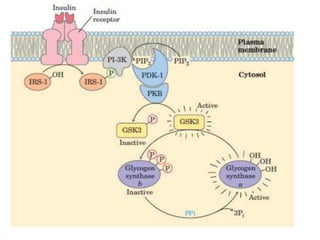
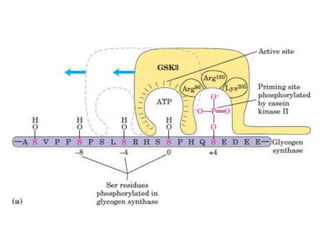
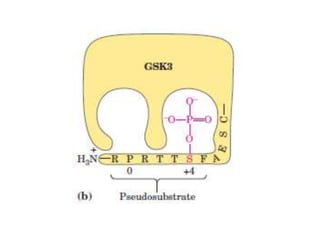
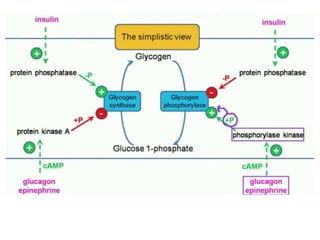
This document discusses glycogen metabolism. It notes that glycogen is the major storage carbohydrate in animals, found mainly in the liver and skeletal muscle. Glycogen is a branched polymer of glucose that is synthesized from glucose-1-phosphate via glycogen synthase. It can be broken down to glucose-1-phosphate by glycogen phosphorylase to maintain blood glucose levels. The activities of glycogen synthase and phosphorylase are regulated by phosphorylation and dephosphorylation in response to hormones like insulin and glucagon to control glycogen synthesis and breakdown.