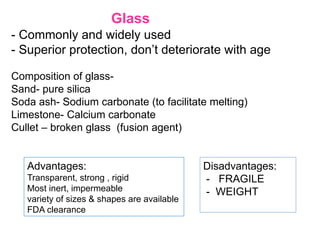
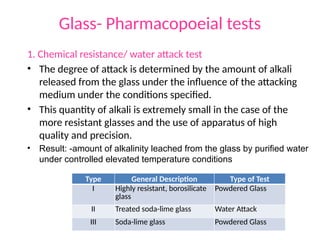
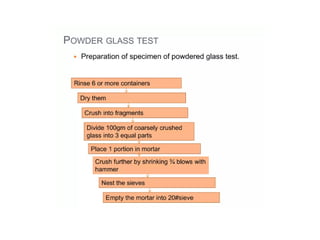
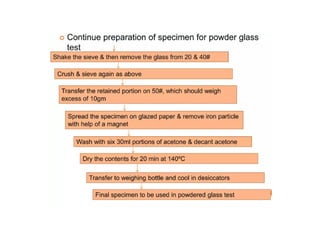
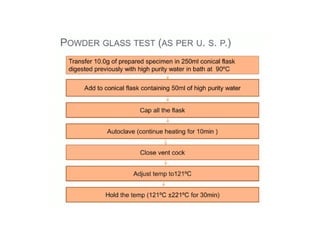
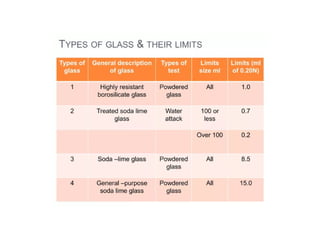
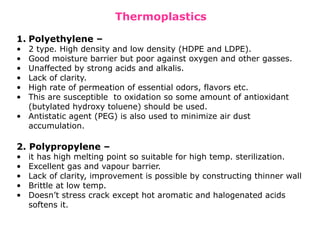
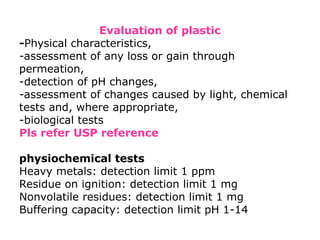
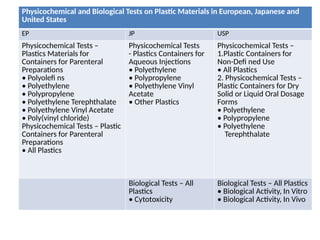
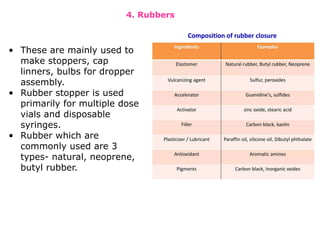
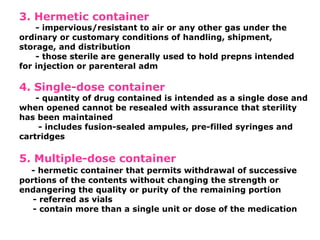
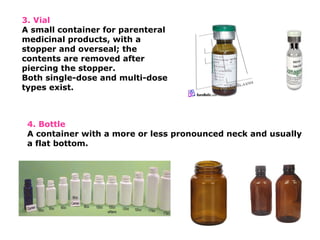
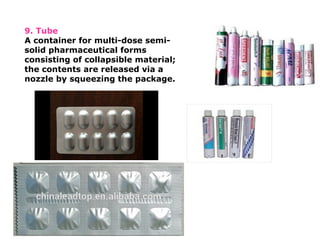
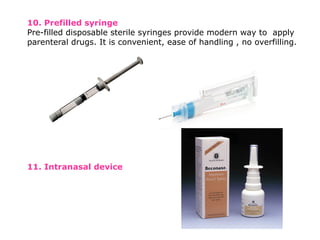
The document provides a comprehensive overview of packaging, particularly within the pharmaceutical industry, detailing its definitions, importance, functions, and the characteristics of various packaging materials such as glass, plastic, and metal. It emphasizes the significance of protection, containment, and tamper evidence, as well as discusses the properties and tests for different types of packaging materials, including mechanical, chemical, and biological assessments. Additionally, it outlines the regulatory requirements, selection criteria, and evaluation methods for packaging materials to ensure their suitability for medicinal products.