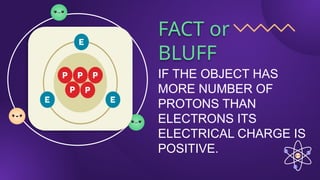
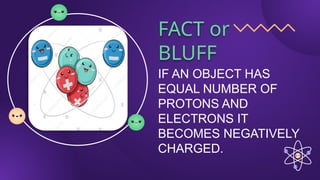
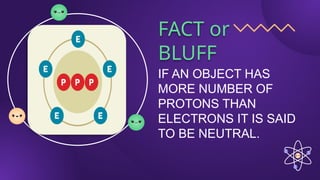
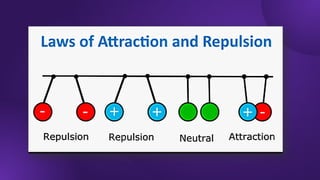
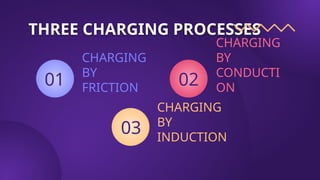
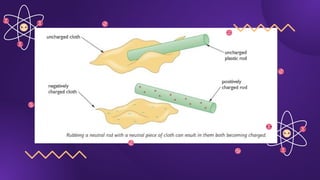
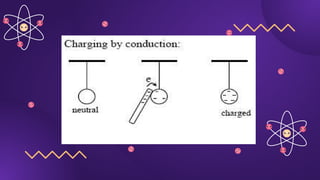
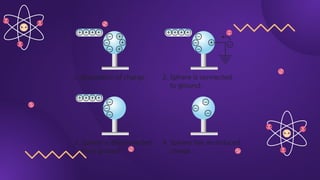
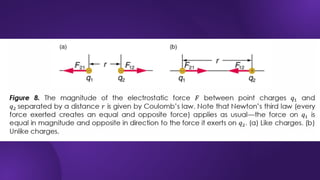
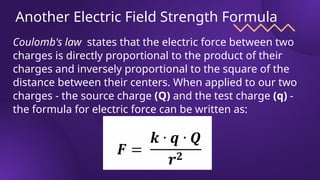
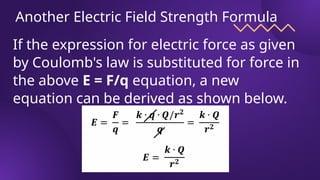
The document covers fundamental concepts of electric forces and fields, detailing the nature of electric charges, conductors, insulators, and charging processes. It explains Coulomb's law, describing the relationship between electric charges and the forces acting between them, as well as the concept of electric fields and how they function at a distance. Additionally, several sample problems illustrate the application of these concepts in calculating electric force and electric field strength.