This document provides an overview of gemstones, including their physical and optical properties, shapes and cuts, and synthetics and simulants. It discusses the key properties used to identify gemstones such as crystal habit, specific gravity, hardness, refractive index, and luminescence. Different cutting styles including cabochon and faceted cuts are described. The document also covers synthetic gemstones which mimic natural stones, and simulants which have similar appearances but different properties. It aims to equip gemologists with the knowledge to distinguish natural gems from synthetics or enhanced stones.









![When white light (wavelengths from ~ 400 to 700 nm) is incident on a gemstone, it may be
transmitted, scattered, reflected, refracted or absorbed. If the light suffers no absorption, the
mineral is colorless. Minerals appear colored when certain wavelengths of light are absorbed.
The perceived color results from the combination of those remaining wavelengths that reach the
eye.
The electronic processes responsible for light absorption and color are as follows:
(i) Crystal Field Transition
Crystal field transitions are electronic transitions between partially filled 3d orbitals of transition
elements. These are the elements of the first transition series with atomic numbers from 22 to 29
and having electronic configuration of the general form 1s2
2s2
2p6
3s2
3p6
3d10-n
4s1-2
. The
electrons in the partially filled d-orbitals can be excited by quanta of energy from the visible
spectrum. Such electronic transitions are the basis for production of color. The transition
elements are therefore called chromophores.
When such chromophoric transition element/elements are the major constituents of the
composition of a crystal/gemstone, then the minerals are described as idiochromatic.
e.g. The red color of almandine garnet (Fe3Al2Si3O12) is attributed to Fe+2
while the blue color of
turquoise [CuAl6(PO4)4(OH)8.5H2O] is due to the dominance of Cu+2
.
In contrast, gemstones are allochromatic when their color is attributed to small amounts of
chromophores present as impurities within the crystal structure. Even less than 0.01% of such
elements would be enough to produce an appreciable perception of color. Table 3.2 lists the
different gem varieties of allochromatic corundum and the relevant chromophores while Table
3.3 is a list of the transition elements in order of increasing atomic number and the minerals
(idiochromatic or allochromatic) to whom they have imparted the color.
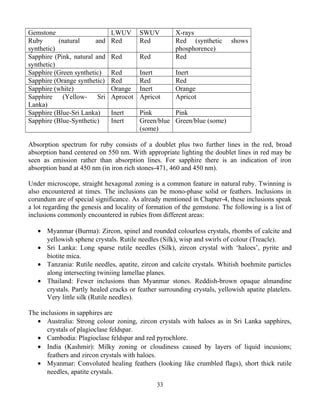
Table 3.2. Gem varieties of corundum and their chromophores
Gem (Corundum) Color Chromophore
1. White Sapphire
2. Ruby
3. Blue Sapphire
4. Yellow Sapphire
5. Padaparadscha
White
Red to pink – red
Blue
Yellow
Orange – pink
Pure
Cr+3
Ti+4
– Fe+2
Fe+2
– Fe+3
Fe+3
+ Fe+2
+ Cr+3
Table 3.3. Transition elements and minerals they have colored
Titanium (Ti) Blue sapphire (with iron), blue zoisite
Vanadium (V) Grossular garnet (Tsavorite), green vanadium beryl,
synthetic corundum (alexandrite simulant), synthetic
emerald (few), blue/violet sapphire
Chromium (Cr) Ruby, emerald, red spinel, pyrope garnet, chrome grossular
garnet, demantoid garnet, uvarovite garnet (idiochromatic))
chrome diopside, green jadeite, pink topaz, alexandrite,
hiddenite (spodumene)
11](https://image.slidesharecdn.com/gemmologynotes-171021164109/85/Gemmology-notes-11-320.jpg)





![In contrast to the color of a gemstone, which is observed in daylight, there are certain minerals,
which produce color or visible light in darkness under certain special circumstances. This
phenomenon is known as luminescence. It is found that when certain materials acquire surplus
energy in one form or another (but below the level of burning or glow) they convert this energy
into a ‘cold’ radiation whose wavelength generally lies in the visible section of the spectrum.
The mechanism producing this ‘cold radiation’ or ‘luminescence’ is associated with the
excitation of atoms within the material. The surplus energy acquired by luminescent substances
is used up in moving electrons out of their normal orbital state (ground state) temporarily into
orbits of a higher energy level (excited state). This high-energy state is unstable so the electrons
relax into a lower energy excited state that is slightly more stable. When these electrons
eventually return to their more stable orbits (ground state) they give up the surplus energy in the
form of electro-magnetic radiations. This emitted energy is always less than the excited energy.
Since wavelength increases as the energy decreases, emission occurs at larger wavelengths than
the excitation wavelengths. For example, stimulus of shorter wavelengths of ultraviolet (less
than ~ 400nm) rays can result in emission of longer wavelengths in the visible range (e.g.
natural ruby frequently give red [~700nm] luminescence under long wave U.V).
In case of gemstones, the best stimulant is the radiation by invisible shorter wavelengths or
ultraviolet rays (UV). UV lamps that produce light of two different wavelengths check
luminescence of gemstones normally.
1. Short wave UV lamp (253.7nm)
2. Long wave UV lamp(365 nm)
Certain minerals respond better to short wave UV radiation while some fluoresce better in long
wave UV radiation.
A substance is fluorescent if the emission of light stops as soon as the energy source causing it
is removed; if it continues to glow even after the source of stimulant is cut off, then it is called
phosphorescence (e.g. Kunzite). Luminescence (fluorescence / phosphorescence) should
always be checked in dark room.
In all forms of luminescence, the light emitted is either due to some intrinsic property of the
material (e.g. lattice defect in diamond) or due to the presence of luminescent impurities called
activators (e.g. Cr2O3 in ruby).
3.2.7. Pleochroism
As pleochroism is an useful identifying property for a gemstones, an instrument called
Dichroscope is used for quick discrimination. It consists of a cleaved rhomb of optical quality
calcite (Iceland spar), which is mounted, in a glass tube having an eyepiece at one end and a
square aperture at the other end. A glass prism is cemented to each end of the calcite rhomb to
allow the light to enter and leave in a straight line. When the colored gemstone, if doubly
refracting and pleochroic, is viewed in direction other than that of an optic axis, the two images,
17](https://image.slidesharecdn.com/gemmologynotes-171021164109/85/Gemmology-notes-17-320.jpg)

















![Topaz [Al2 (F, OH)2 SiO4] crystallizes in the orthorhombic system and has a prismatic habit with
pyramidal terminals. It is most often colorless but can also occur in shades of yellow, blue and
even pale green. Pink and blue topaz can also be produced by several enhancement techniques.
The luster is vitreous. Hardness of topaz is 8 in Moh’s scale. The R.I. and SG are 1.63-1.64 and
3.53 for yellow and pink stones while it ranges in 1.61-1.62 and 3.56 for blue and colorless ones.
Double refraction varies from 0.01 in colorless, brown, blue and yellow topaz to 0.008 in pink
and orange varieties. Optic sign is positive. Sherry-brown or golden-yellow topaz from Brazil
contains traces of chromium and shows an orange fluorescence under LW UV light. In pink
heat-treated stones the fluorescence is stronger and redder. Under spectroscope a thin line in red
band at 683.8 nm is seen for such pink stones. Under microscope, mineral inclusions and two-
phase fluid inclusions are at times observed. Topaz has a perfect basal cleavage which plays an
important role during gem cutting.
A common stimulant of topaz is yellow quartz or citrine. However, it’s physical and optical
properties (H = 7; SG = 2.65 and R.I. = 1.54-1.55) are distinctly different from yellow topaz (H
=8; SG = 3.53 and R.I. = 1.63-1.64). Besides, the former crystallizes in trigonal system and the
later in orthorhombic which can readily be recognized in uncut crystals. Apart from quartz, other
yellow stones are yellow sapphire, yellow tourmaline, chrysoberyl, rutile and zircon. Pink
tourmaline and pink sapphire can be confused with pink topaz while pale blue-green topaz is
often mistaken for aquamarine. However, all these yellow minerals have distinctly different SGs
and R.I.s and can be readily identified with preliminary gemological tests.
Counterfeits like pastes with similar refractive indices and synthetic sapphires are at times sold
as topaz. Such counterfits will not show any double refraction and have different SGs. Doublets
are rare. Synthetics of topaz are not prepared commonly due to its complex composition.
Gem variety topaz mostly comes from Brazil.
7.1.7. Quartz, Chalcedony, Tiger’s Eye and Opal
Quartz is the commonest and yet one of the most beautiful of all minerals. Compositionally, it is
pure silica (SiO2). It crystallizes in the trigonal system and has a hexagonal pyramidal habit
terminating in positive and negative rhombohedra. Fine twinning related striations occur at right
angles to the c-axis. However, untwined quartz free of striations can also be encountered. Quartz
occurs in a variety of colors: it can be colorless, transparent (rock crystal), milky white, pale to
dark purple (amethyst), yellow (citrine), bi-colored with purple and yellow (ametrine), pink
(rose quartz), brown to blackish, translucent (smoky quartz), blue, translucent to opaque (caused
by rutile needles) and even green (prasiolite). Amongst these varieties, the rock crystal,
amethyst, citrine and rose quartz are popularly used as semiprecious gemstones. The crystalline
quartz have H = 7, SG = 2.65 and R.I. = 1.544 and 1.553, DR = 0.009, vitreous luster and
conchoidal fracture. They float in bromoform. Quartz is uniaxial positive.
Rock crystal (sphatik) is frequently used for making beads. It has both aesthetic and religious
appeal in India as well as in other parts of the world. Under microscope, it will generally contain
a few fluid inclusions. The common stimulant is glass. However, gas bubbles and single R.I. can
39](https://image.slidesharecdn.com/gemmologynotes-171021164109/85/Gemmology-notes-39-320.jpg)


![Opals can be synthetically produced. Gilson synthetic opal fluoresce a dusty green under SW
UV. Opals simulants are produced from polystyrene latex and coated with acrylic coating in
Japan These have a much lower SG of 1.2 and higher R.I. of 1.51. They are also hydrophobic (a
drop of water placed on the surface forms a hemispheric bead) in contrast to natural opals that
are hydrophilic (water spreads rapidly along their surface). Glass imitations of opal (Slocum
stone) has SG = 2.4-2.5) and R.I. = 1.49-1.51. Also bubbles and swirl marks may be present in
these stones.
7.1.9. Jade
Jade is a term which the mineralogists consider permissible for two distinct minerals: jadeite or
‘Chinese jade’ is the rarer and precious verity while nephrite is the other variety also used
widely (Plate-3).
Jadeite is a sodium aluminium silicate [NaAl(SiO3)2] and belongs to the clinopyroxene group of
minerals. The gem variety jadeite is basically a polycrystalline aggregate of bladed and fibrous,
randomly oriented grains. The color of green jadeite varies in saturation from palest shades to
deep emerald hue. Mottled, patchy colors are common in shades of green and white. The
mineral can also occur in shades of shades of white, mauve, orange, brown and even black. The
better quality jadeites are translucent to semitranslucent. Luster is vitreous to greasy. The
hardness of jadeite is about 6.5-7.0. The SG is 3.30-3.36 (~ 3.33) while the R.I. is 1.654 to
1.667. The absorption spectrum of jadeite gives the diagnostic line at 437 nm in the blue;
chrome-rich green jade has a double in the red, and two bands in the red-yellow, Stained jadeite
has band in the orange and one in the yellow – green (plus the diagnostic line at 437 nm)
Nephrite is calcium bearing ferromagnesian amphibole that was widely used by the ancient
Chinese to curve their beautiful jade pieces. Nephrite is green in color but can as well be opaque
white, grey, yellowish-brown and even black. The SG of nephrite range between 2.90-3.03. The
R.I. is 1.62. Hardness is 6.5 on Moh’s scale. Luster is greasy to vitreous. A weak line in 498 nm
can at times be observed in absorption spectrum.
Jade has its own simulants a few of which have been listed in the table below:
Table : Jade and some of its simulants
Gemstone R.I.. SG H
Jadeite 1.654-1.667 3.30-3.36 6.5-7.0
Nephrite 1.62 2.90-3.03 6.5
Hydrogrossular 1.74-1.75 3.60-3.67 7.0-7.5
Bowenite 1.56 2.58-2.62 4.0-5.0
Amazonite 1.52-1.54 2.56 6.0
Verdite 1.58 2.80-2.99 3.0
Prehnite 1.61, 1.64 2.88-2.94 6.0
Sassurite 1.57-1.70 3.00-3.40 6.5
7.2. Gemstones as rocks
42](https://image.slidesharecdn.com/gemmologynotes-171021164109/85/Gemmology-notes-42-320.jpg)