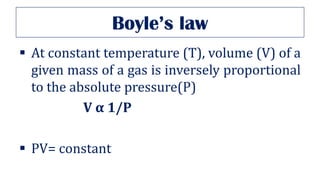
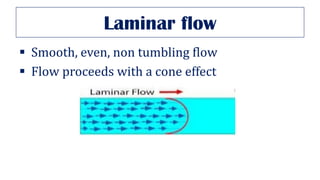
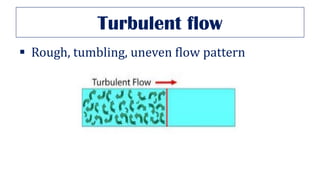
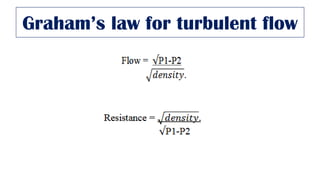
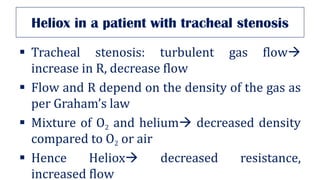
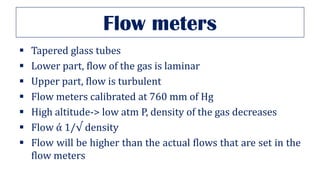
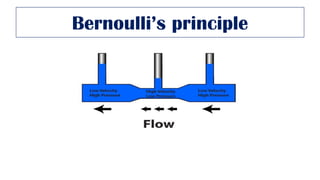
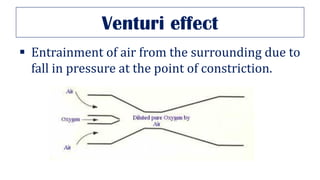
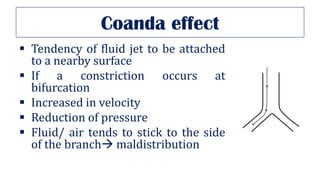
This document provides an overview of various gas laws and their applications in anesthesia. It discusses Boyle's, Charles', and Gay-Lussac's laws, as well as Avogadro's law, Dalton's law of partial pressures, the universal gas law, Hegan-Poissuilles law, Graham's laws of diffusion and effusion, Reynolds number, Bernoulli's principle, Venturi's effect, the Coanda effect, critical temperature, Poynting effect, Henry's law, and Raoult's law. Examples are given on how these principles apply to oxygen cylinders, nitrous oxide cylinders, gas flow and resistance, and the cost of inhalational agents.