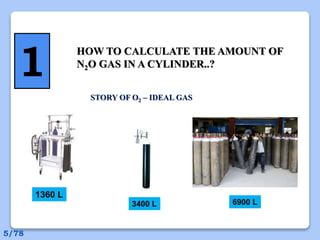
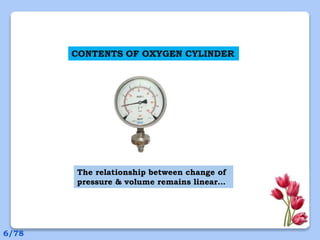
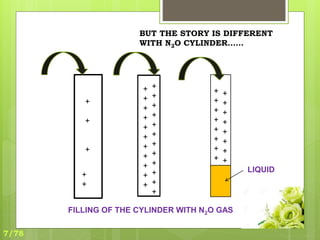
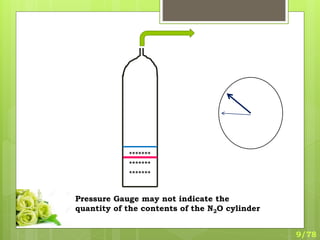
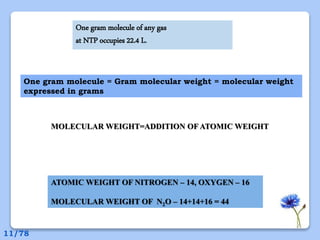
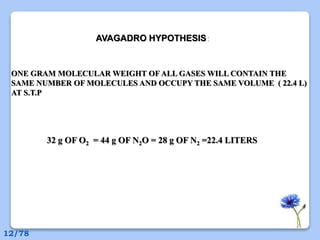
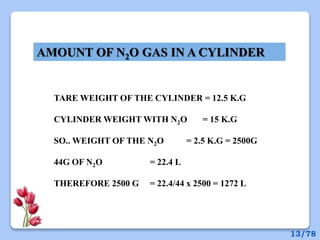
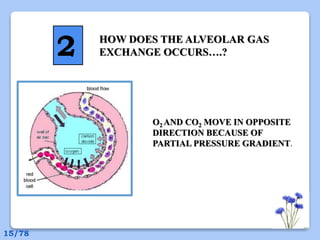
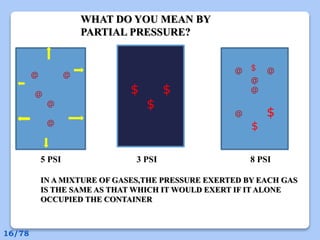
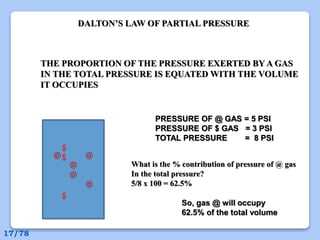
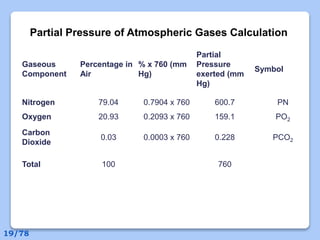
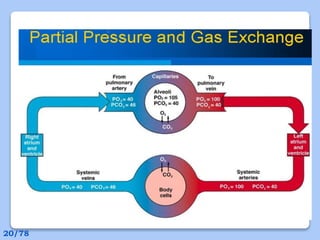
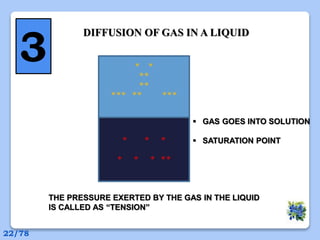
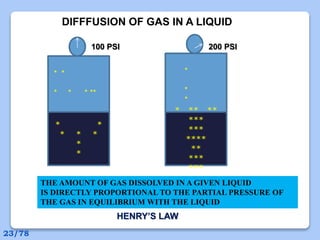
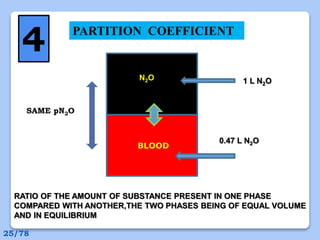
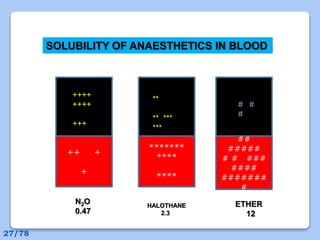
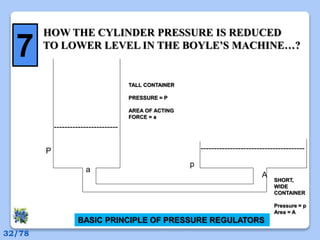
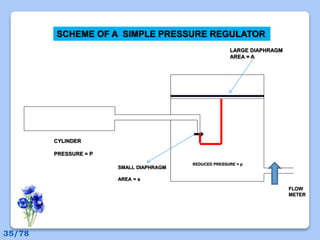
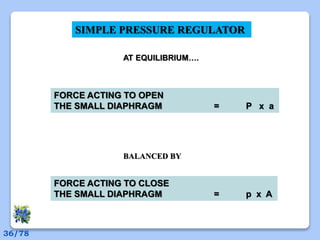
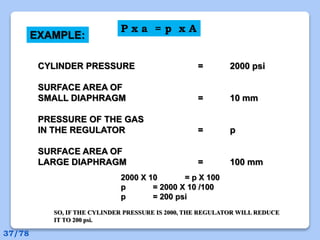
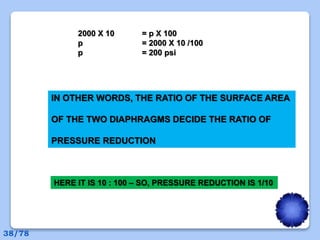
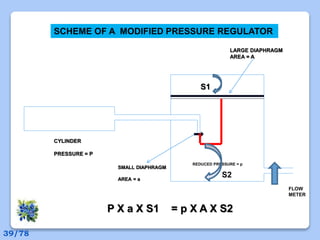
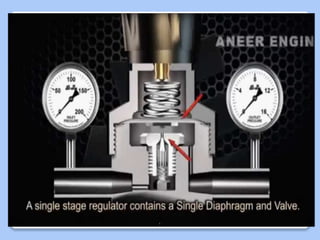
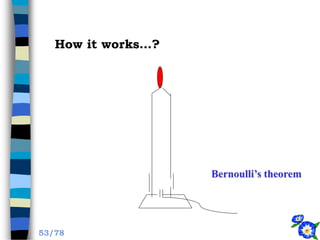
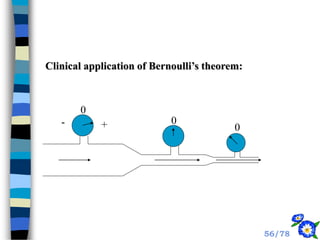
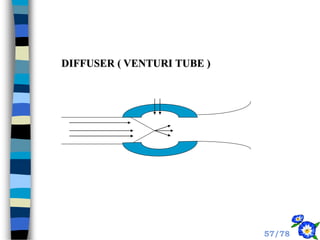
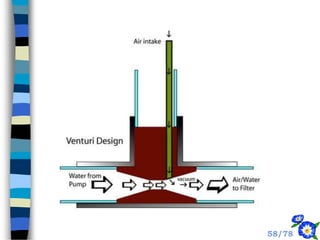
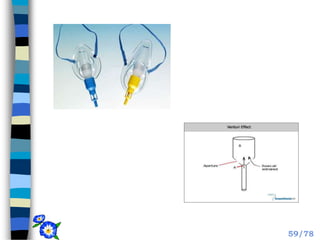
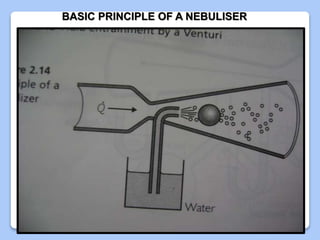
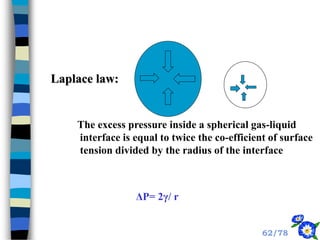
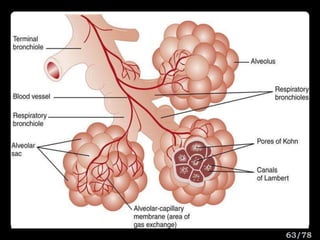
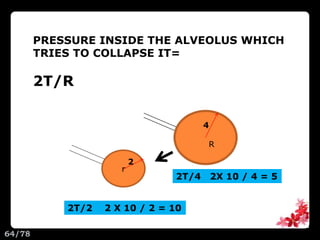
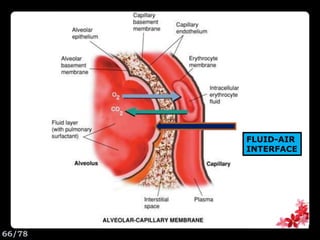
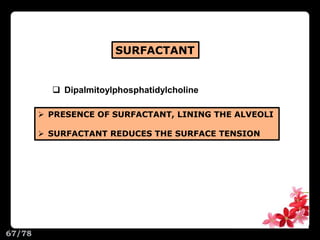
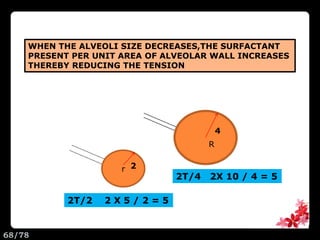
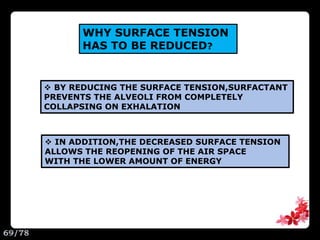
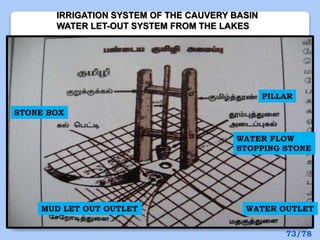
This document discusses the basic physics principles relevant to anesthesiology. It explains how anesthesiologists can calculate the amount of gas in a nitrous oxide cylinder using Avogadro's principle and the cylinder's weight. It also describes how pressure regulators work by balancing the pressure and surface area of two diaphragms. Additionally, it covers partial pressure gradients, diffusion of gases, the Joule-Thomson effect, ventilation principles, and how pulmonary surfactant prevents alveolar collapse.