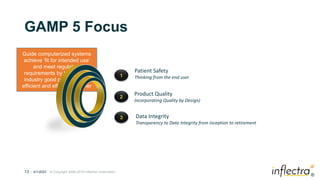
The webinar, led by Dr. Sriram Rajagopalan, discusses the integration of Good Automated Manufacturing Practice (GAMP) compliance into digital health software, focusing on risk management and the software development life cycle. It outlines the significance of GAMP in drug development, emphasizing the need for quality assurance and regulatory adherence throughout various stages. Key topics include the evolution of testing, types of quality activities, and how GAMP can be applied across different industries.