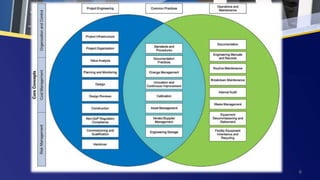
Good Engineering Practice (GEP) encompasses standards, guidelines, and methods for designing, constructing, and operating pharmaceutical and biotechnology facilities, while ensuring compliance, safety, and efficiency. Key components of GEP activities include project engineering, risk management, and cost management, alongside practices such as change management and continuous improvement. A comprehensive GEP approach can greatly enhance the overall operation and lifecycle management of pharmaceutical systems and processes.




















![ BASIC RISK MANAGEMENT TOOLS
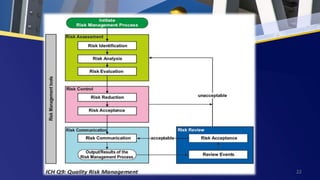
• The Pharmaceutical industry and regulators can assess and manage the risks by
using recognized management tools.
• Below is the non-exhaustive list of some of the tools.
Failure Mode Effects Analysis [FMEA]
Failure Mode, Effects and Criticality Analysis [FMECA]
Fault Tree Analysis [FTA]
Hazard Analysis and Critical Control Points [HACCP]
Hazard Operability Analysis [HAZOP]
Preliminary Hazard Analysis [PHA]
23](https://image.slidesharecdn.com/gep-180122084222/85/GOOD-ENGINEERING-PRACTICES-23-320.jpg)












