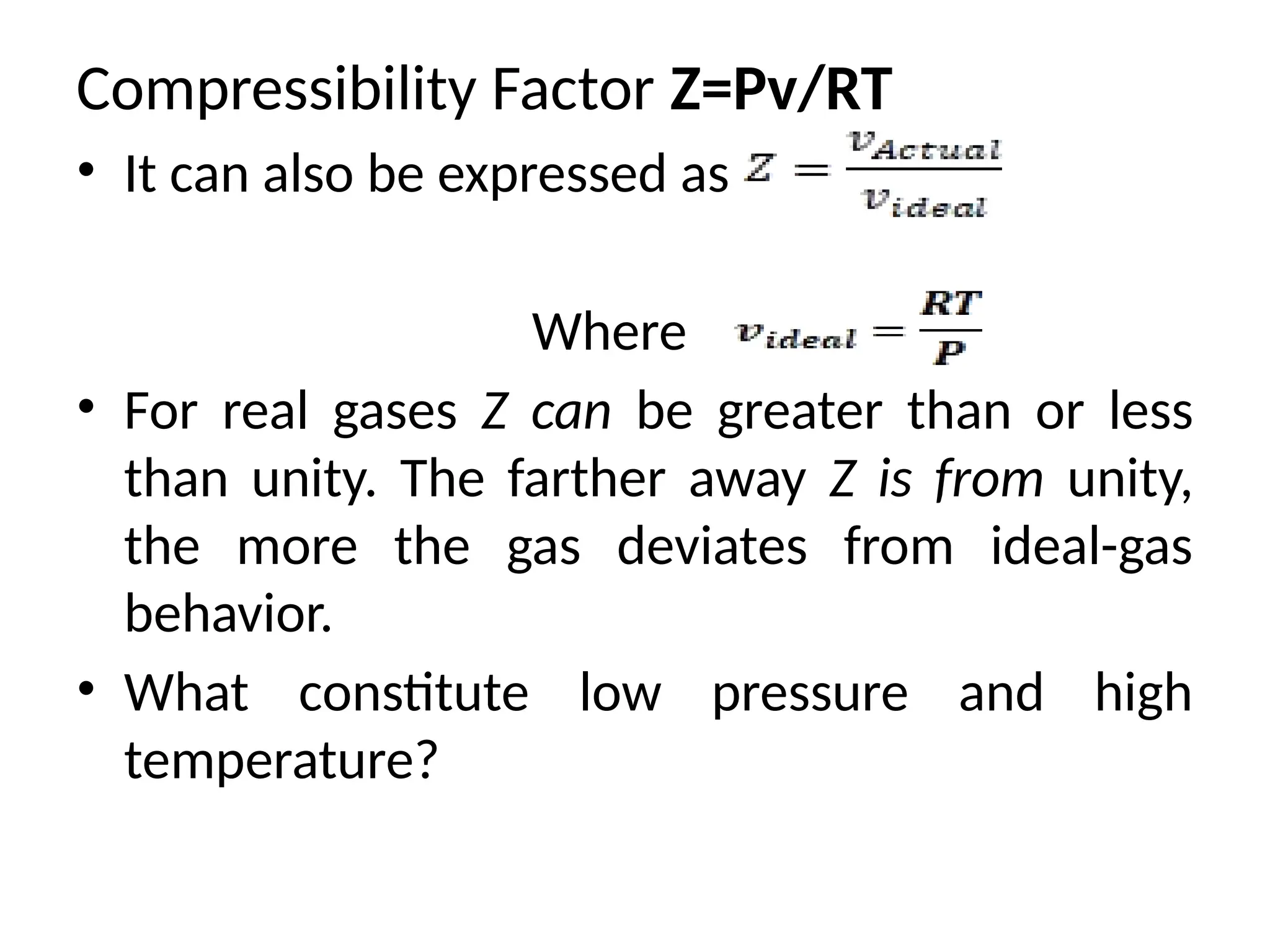
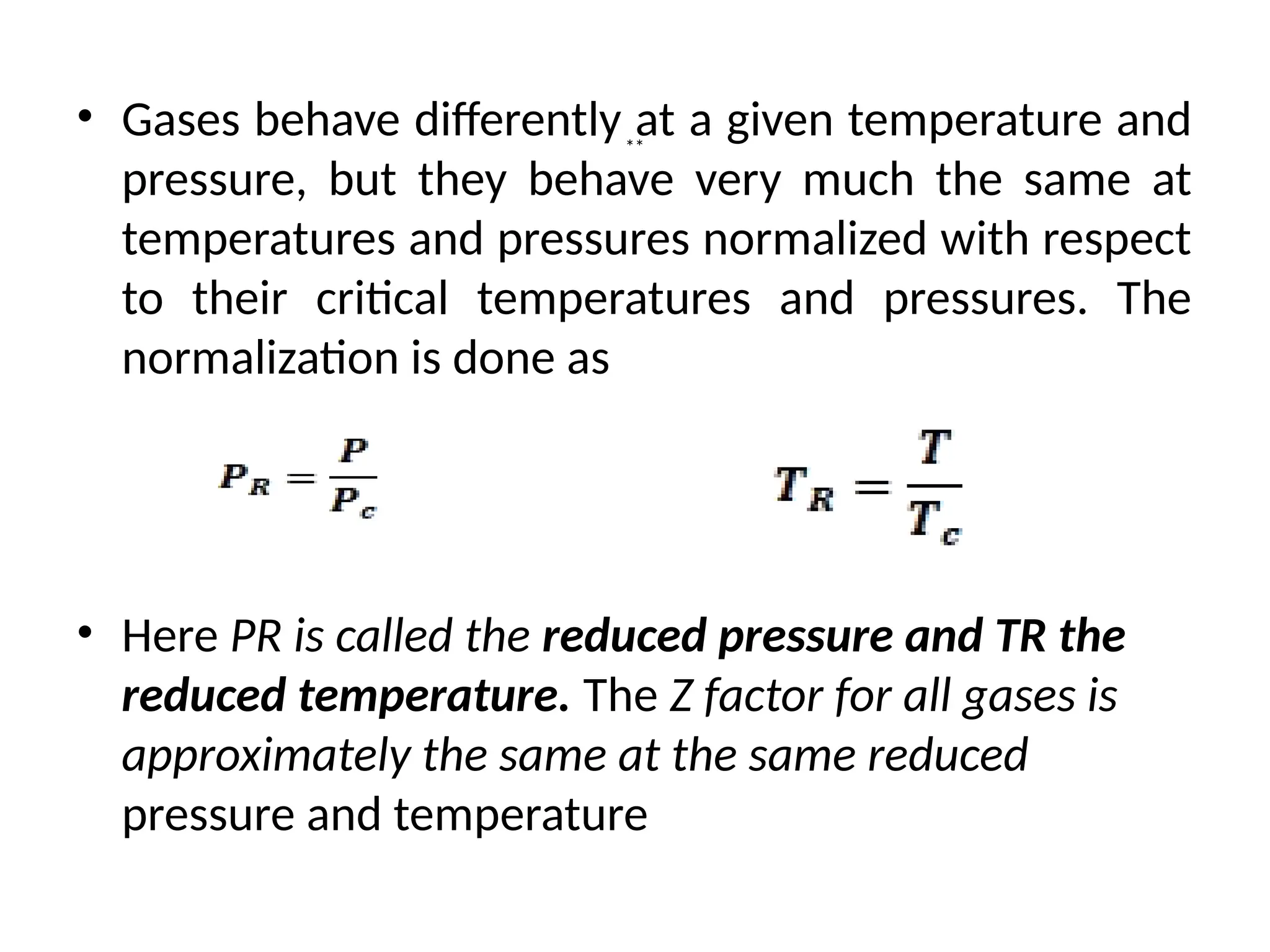
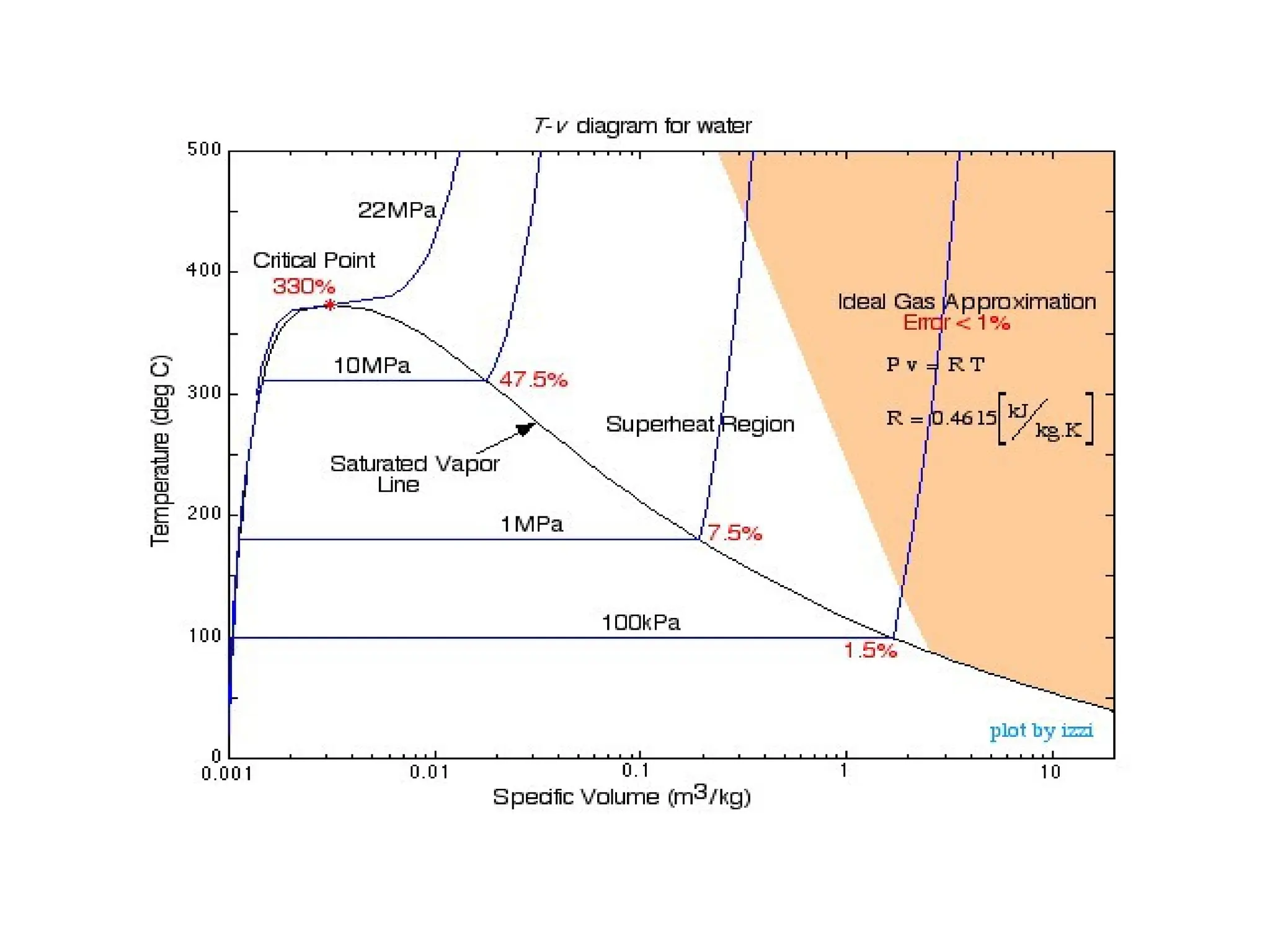
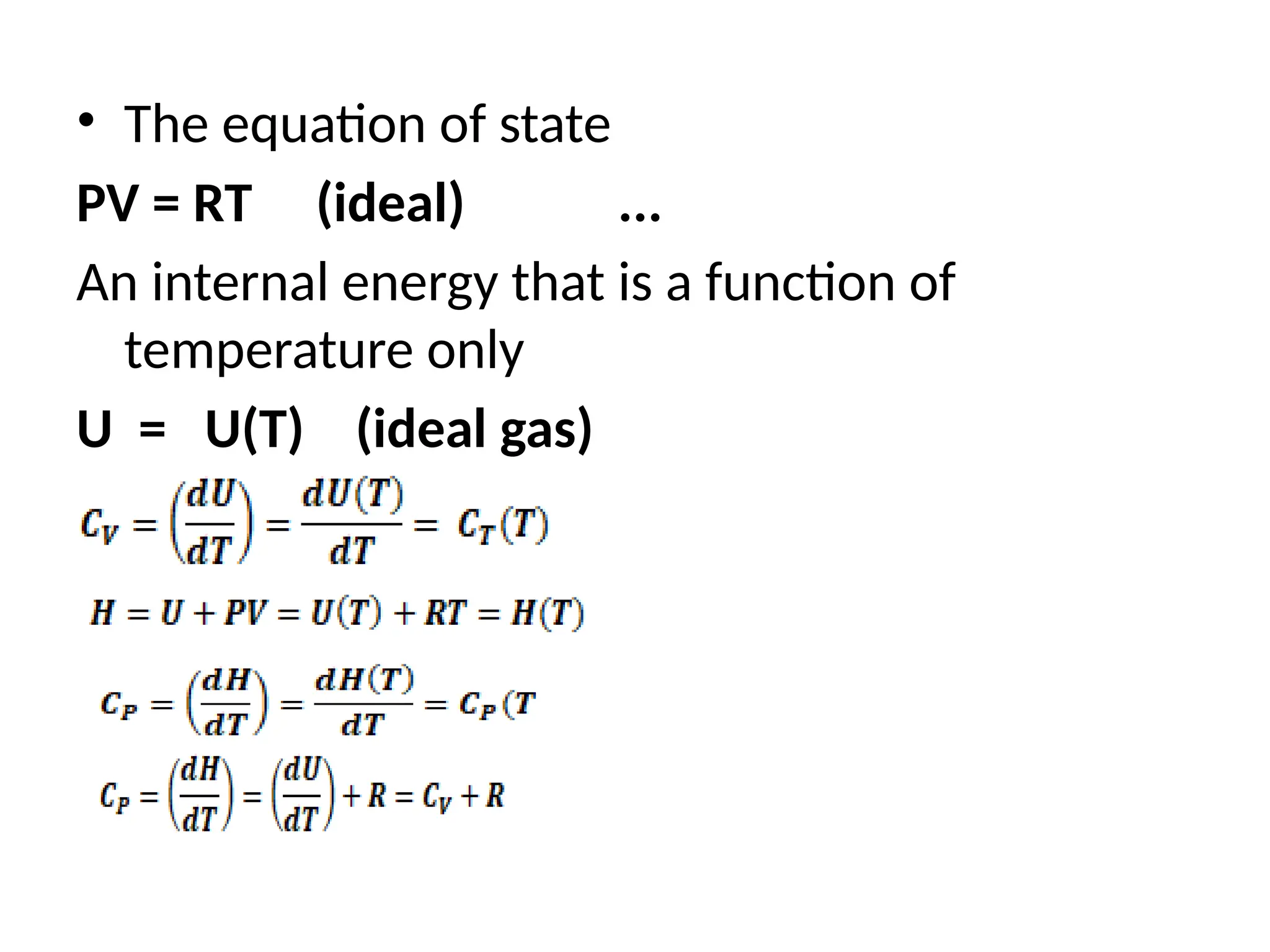
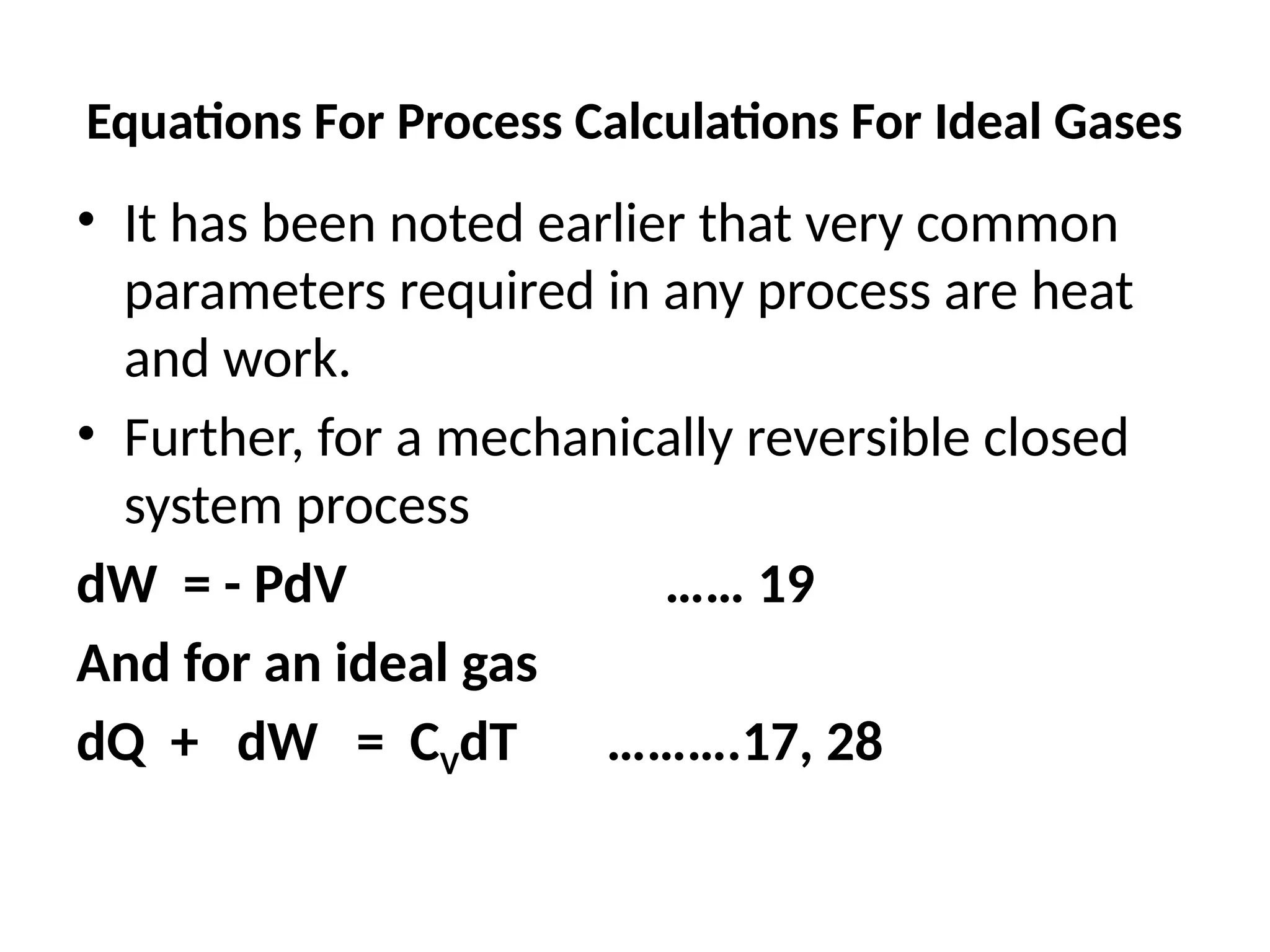
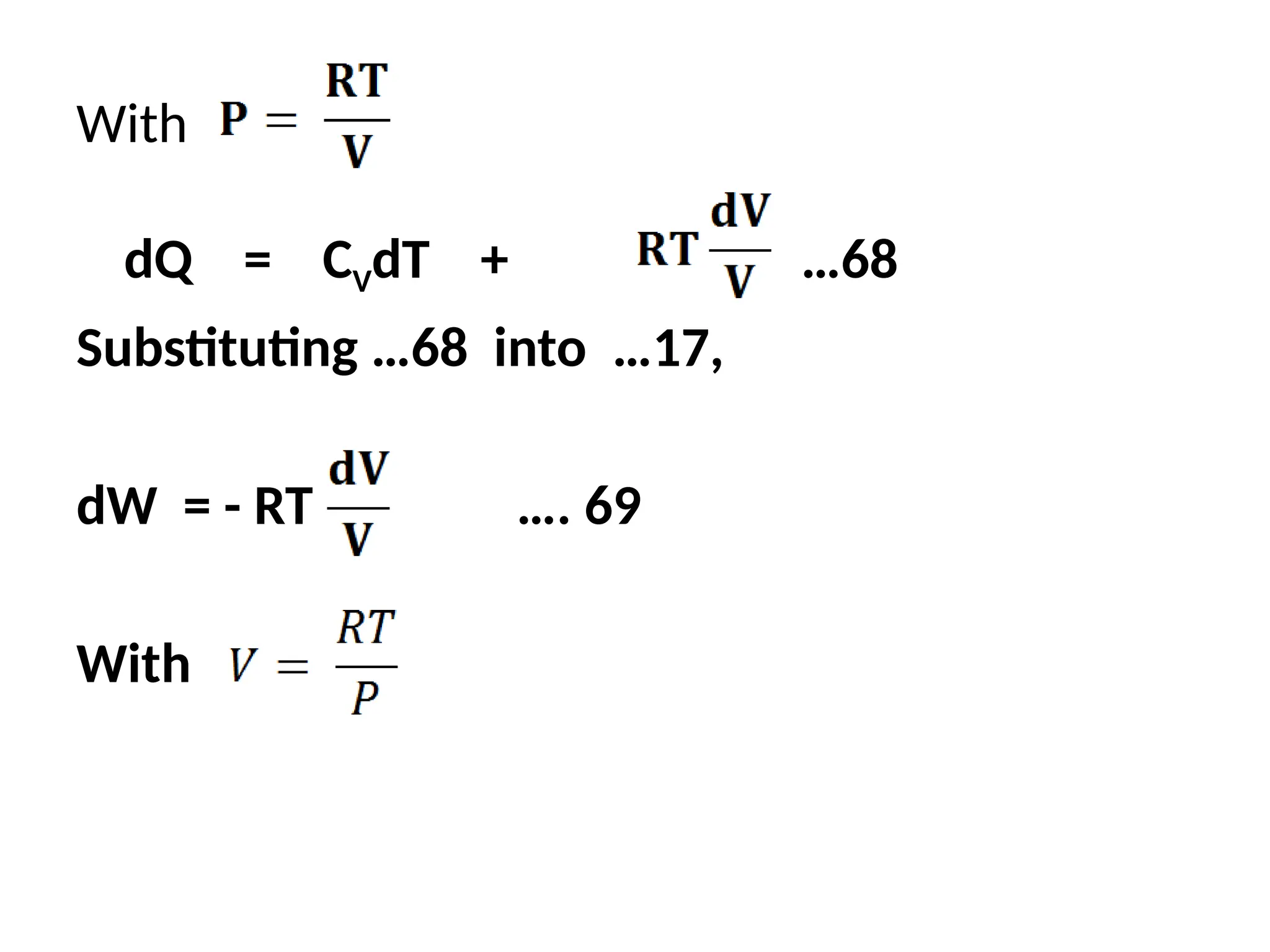
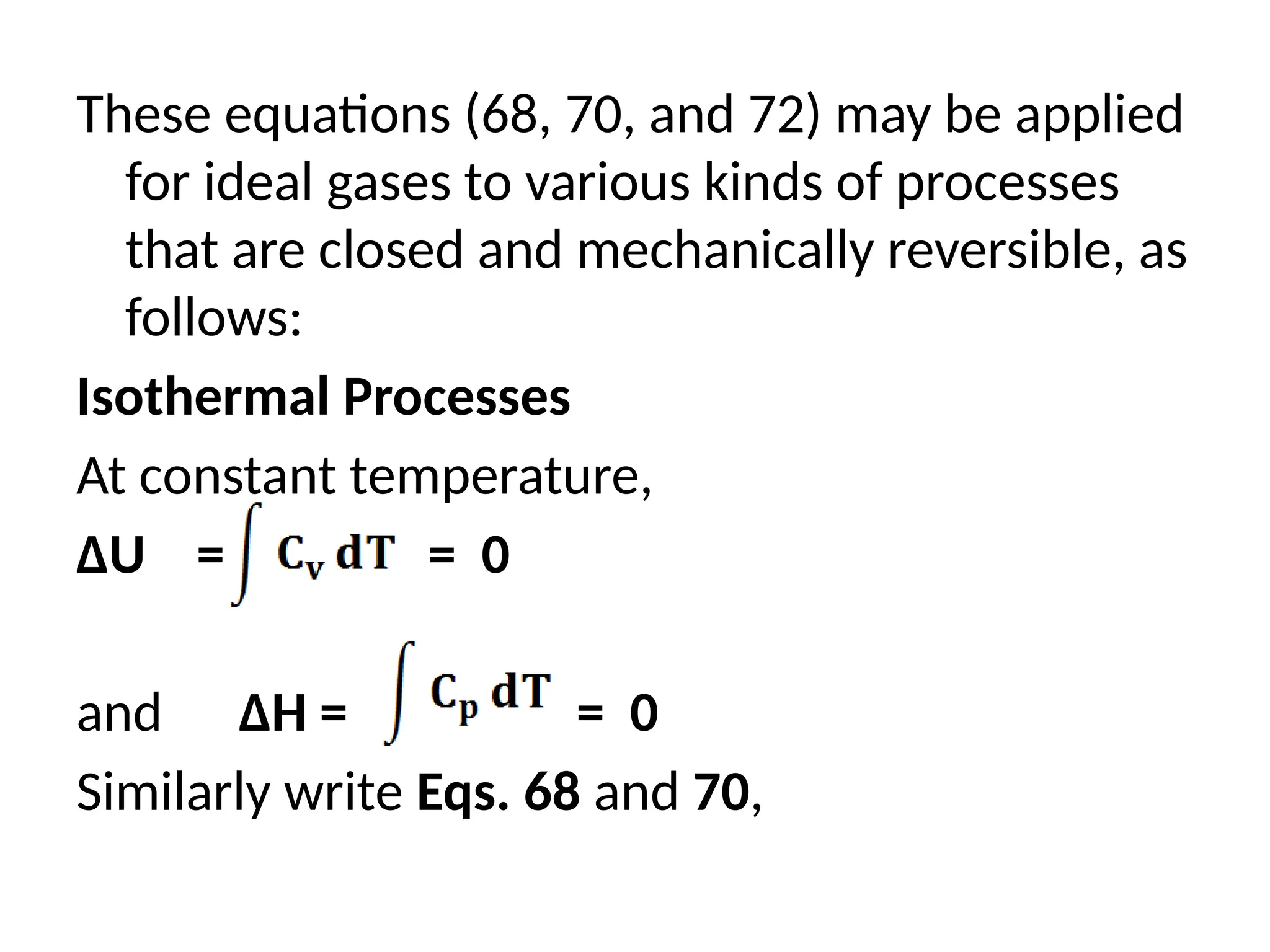
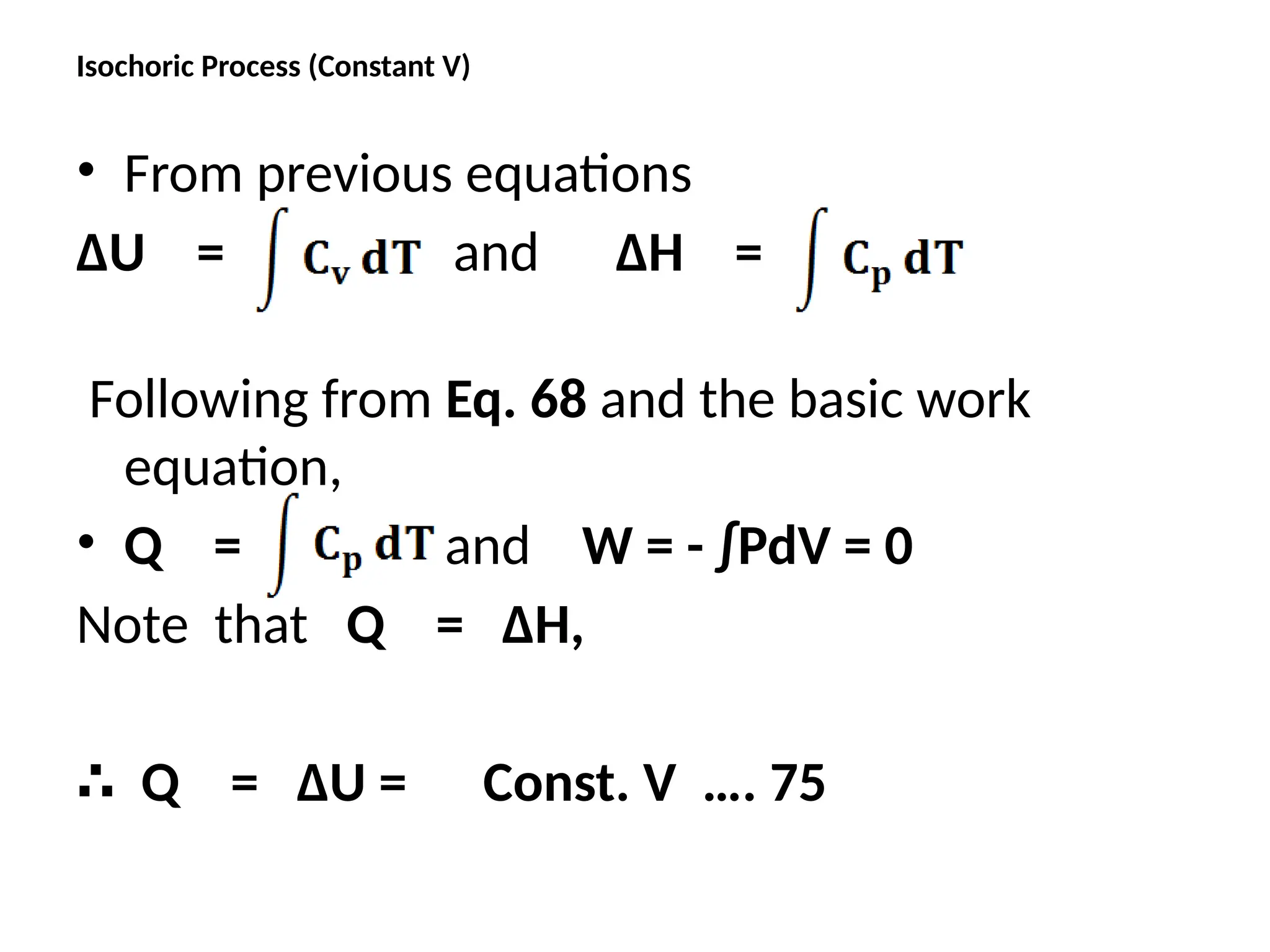
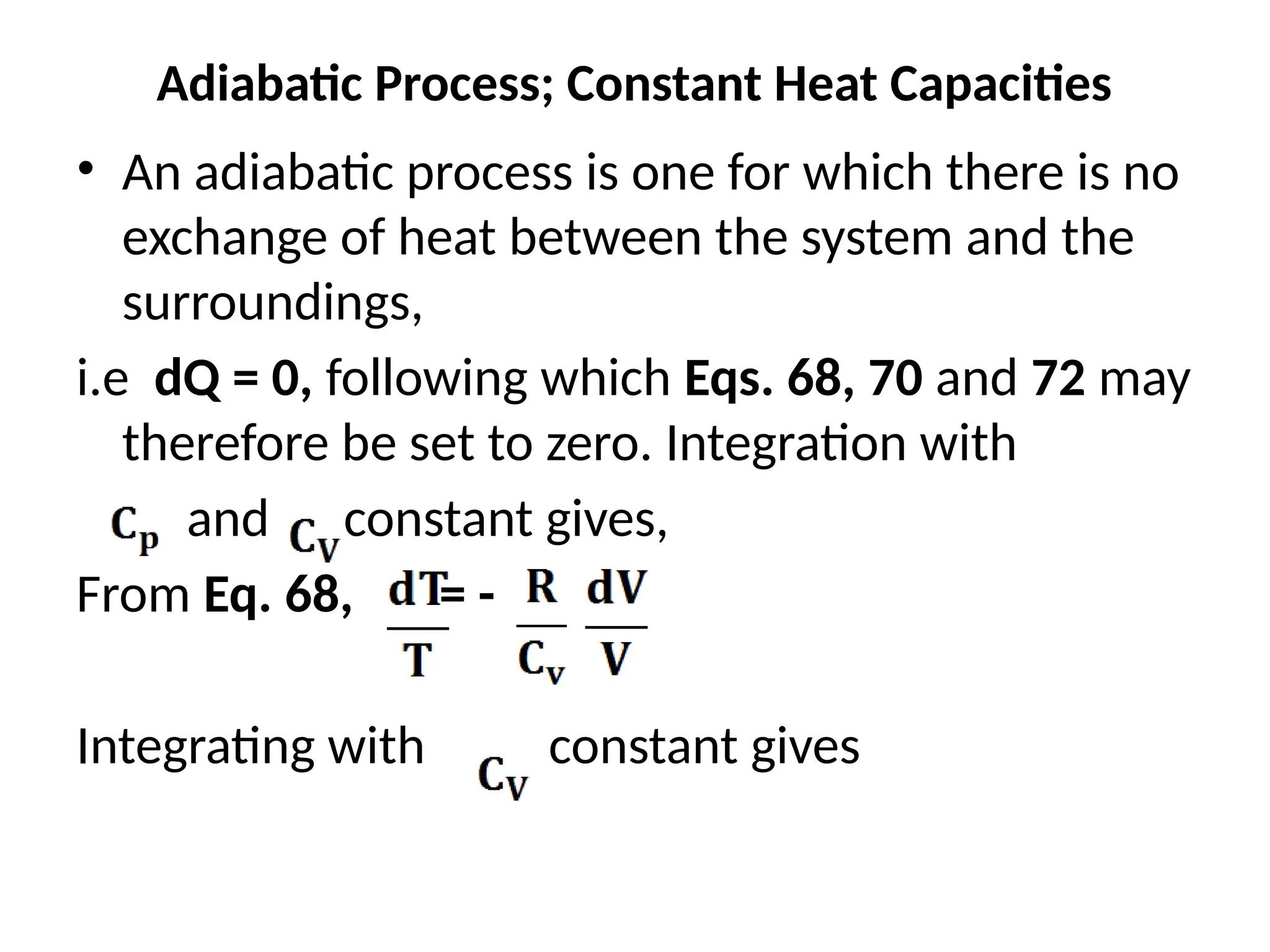
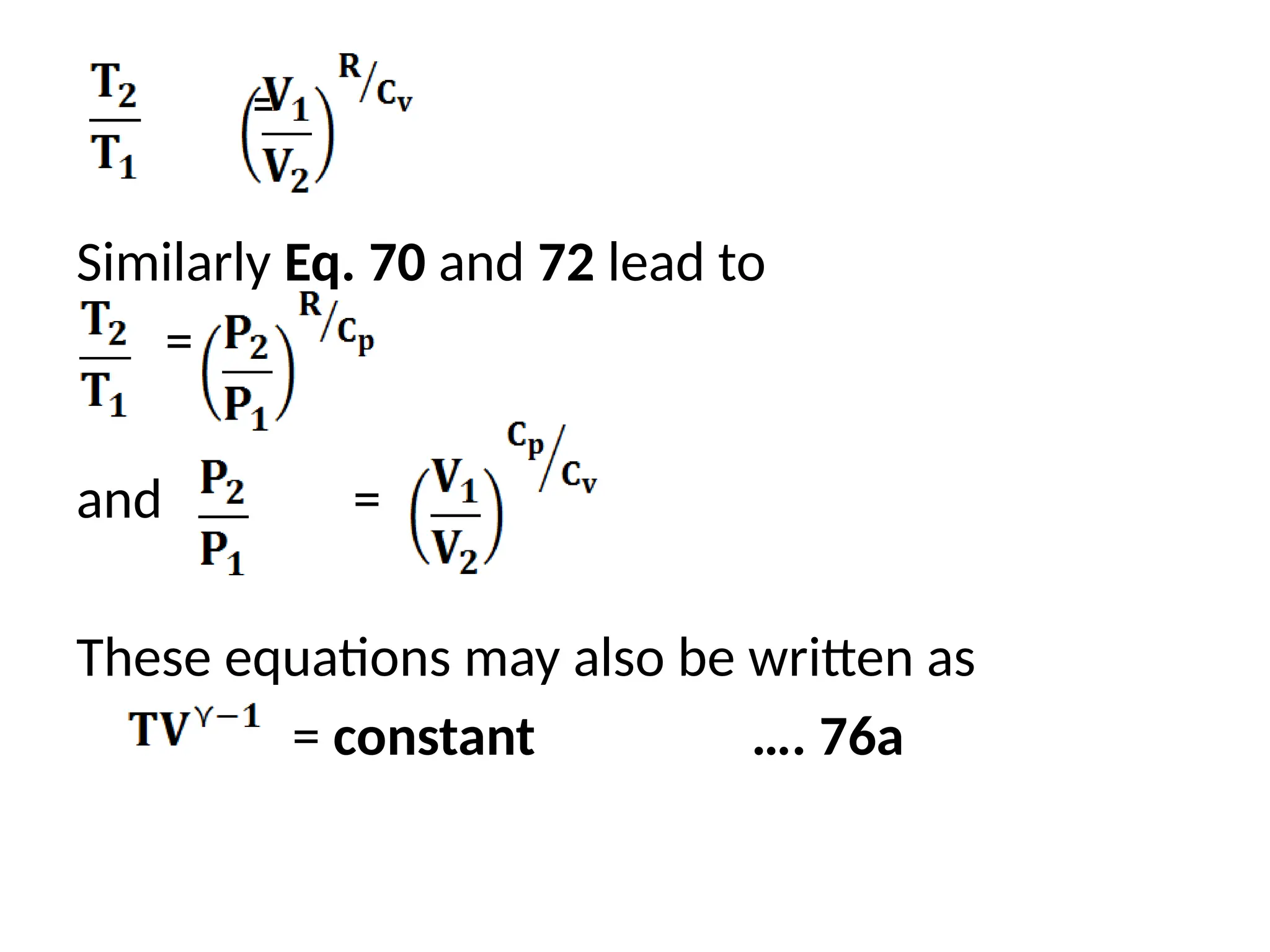
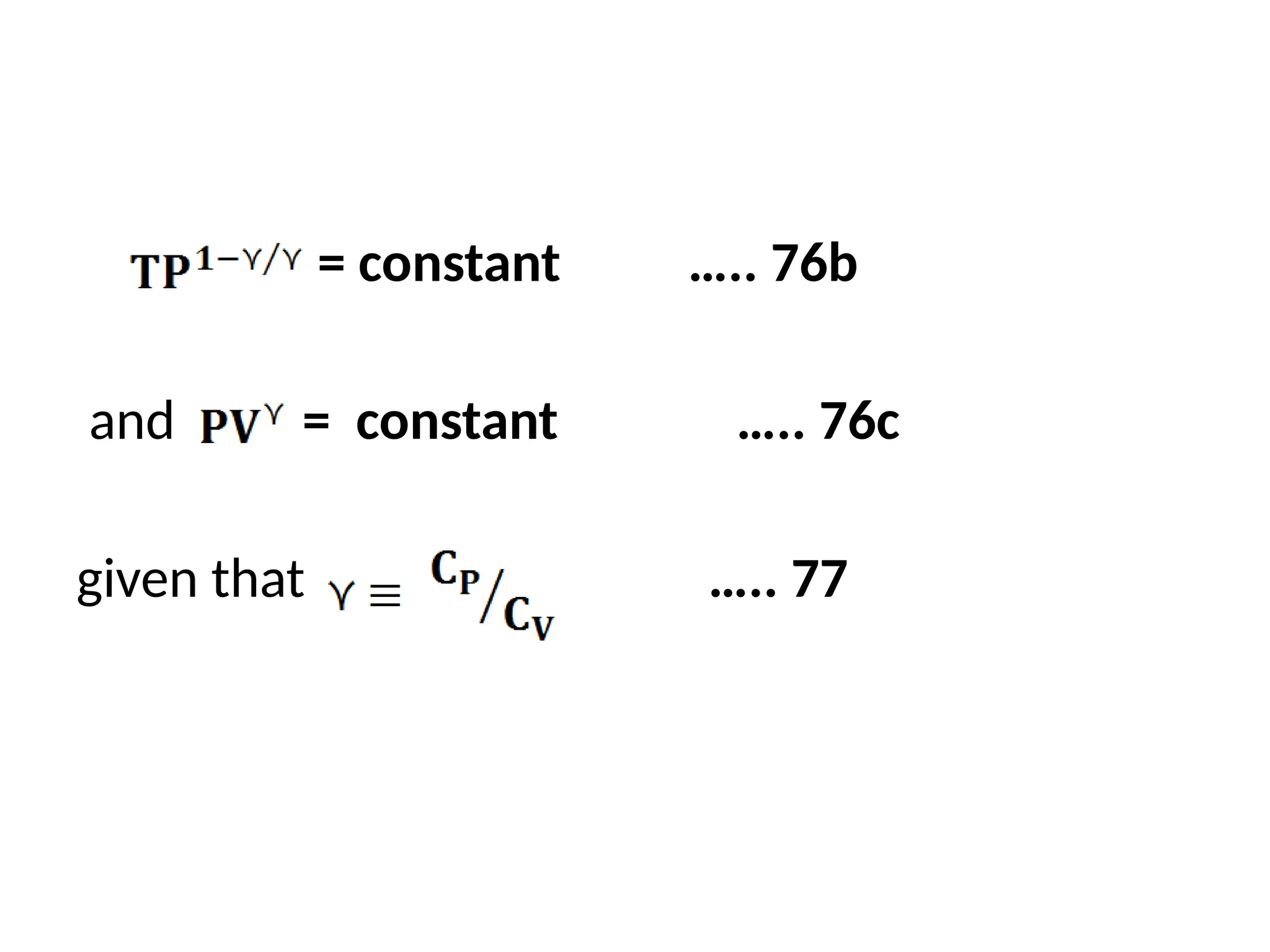
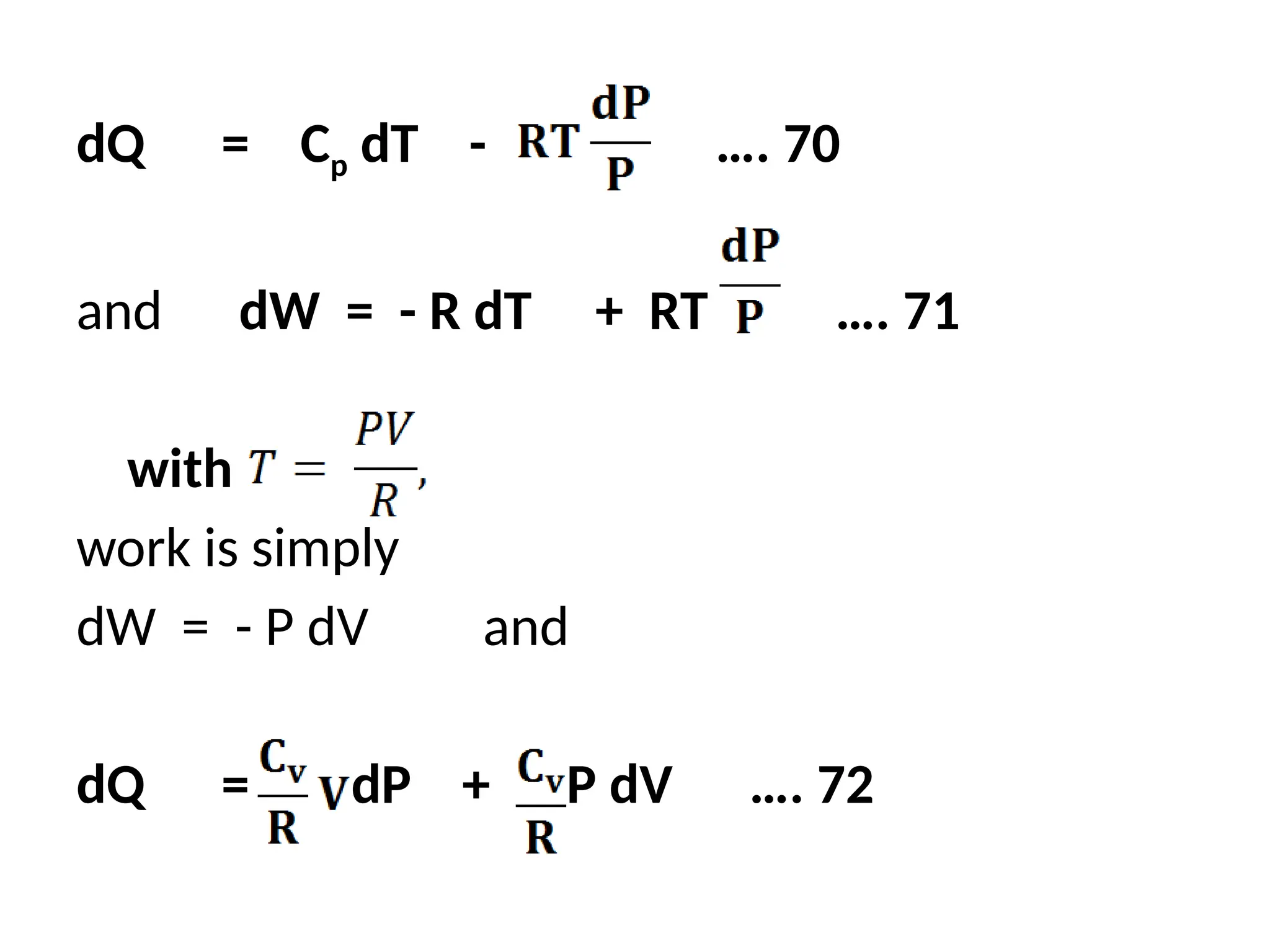The document discusses the properties of ideal gases and their equation of state, particularly the ideal-gas equation pv = rt, which accurately predicts gas behavior under certain conditions. It introduces the compressibility factor z to account for deviations from ideal behavior, especially near critical points or saturation regions. Various equations for process calculations are also presented, focusing on thermodynamic processes involving ideal gases.