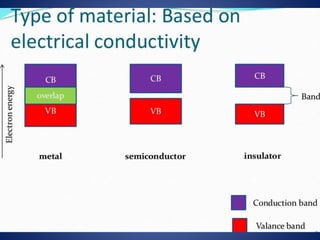
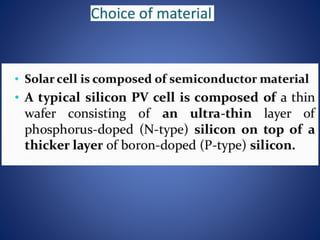
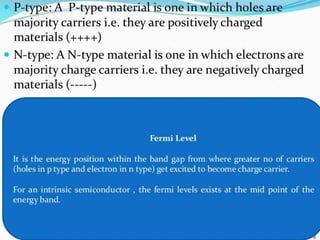
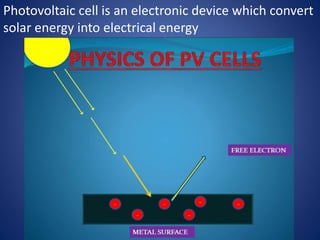
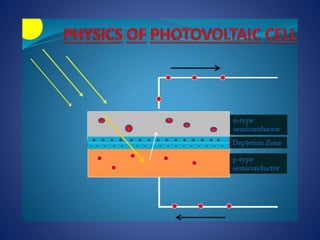
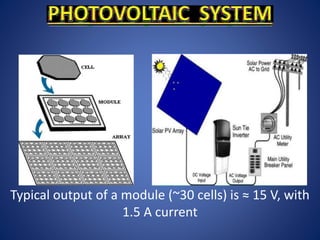
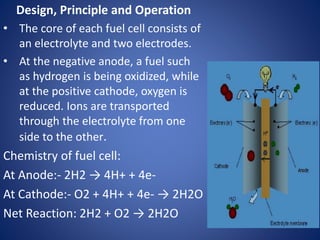
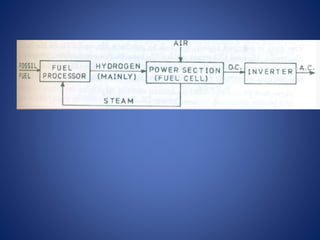
The document discusses direct energy conversion technologies, focusing on photovoltaic cells and fuel cells. Photovoltaic cells convert solar energy into electrical power for applications like water pumping, commercial lighting, and residential power. Fuel cells generate electricity through chemical reactions using hydrogen and oxygen, offering a non-polluting power source, though they face challenges like higher initial costs and difficulties in fuel handling.