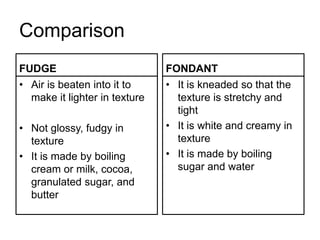
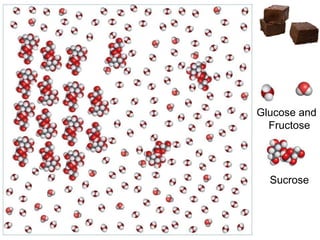
Fudge is a non-crystalline or amorphous sugar candy that is soft and smooth in texture rather than hard or brittle. It is made by carefully controlling the crystallization of sugar when boiling milk, butter, unsweetened chocolate, and sugar to 115°C. Stirring the fudge while it cools promotes many small sugar crystals to form, preventing large crystals that would make the fudge gritty. Fudge has a firm yet smooth texture due to tiny microcrystals in the candy.


![What is a fudge?
• Fudge is a crystalline candy….Crystal
formation is desirable
• Tiny micro crystals in fudge are what give it
the firm but smooth texture.
• It is usually made from
milk
butter
unsweetened chocolate
about 85% sugar
• And cooked till 115°C
• End point separates fudge from caramel
[118-120°C]](https://image.slidesharecdn.com/fudges-180122161133/85/Fudges-characteristic-features-and-principles-4-320.jpg)




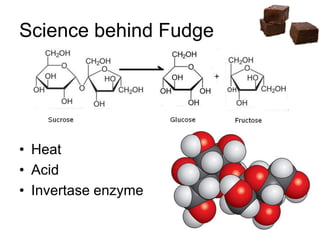
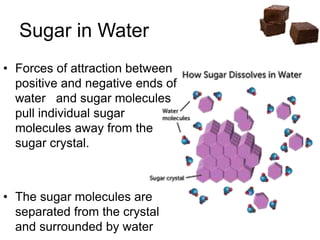
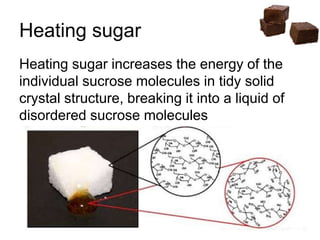


![Cooling
• Heat till soft ball stage [112-115 °C] and
then cool, undisturbed
• Syrup reaches super saturation during
cooling to 46°C
• Tendency to crystallise is stronger
• Stirring
…. Increase seed crystal formation
…. Helps sucrose molecules to find each
other and crystallise](https://image.slidesharecdn.com/fudges-180122161133/85/Fudges-characteristic-features-and-principles-15-320.jpg)