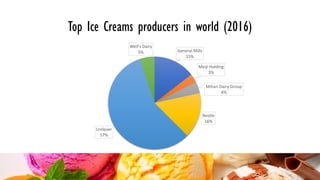
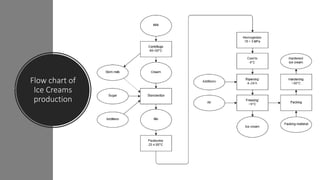
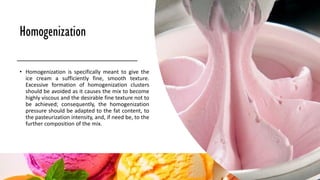
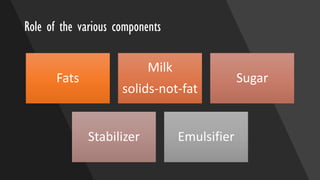
Ice cream is a sweetened frozen food made from dairy products like milk and cream that is typically flavored. It is produced by mixing ingredients, homogenizing the mixture to incorporate air, pasteurizing it to kill microorganisms, freezing it while incorporating more air, and then hardening it to a semi-solid form. Major ice cream producers include Unilever, Nestle, and General Mills. Key components like fat, milk solids, sugar, stabilizers, and emulsifiers contribute to ice cream's texture, consistency, flavor, and shelf life.