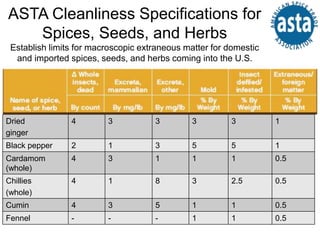
The American Spice Trade Association (ASTA) is a trade organization that represents the U.S. spice industry. It works to ensure spices are clean and safe by establishing cleanliness specifications and providing education and resources to support food safety efforts. ASTA's members include companies involved in importing, growing, processing and marketing spices. It advocates for policies that affect the spice industry and aims to strengthen food safety practices throughout the global supply chain.