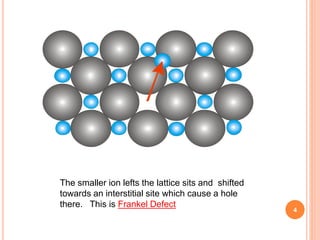
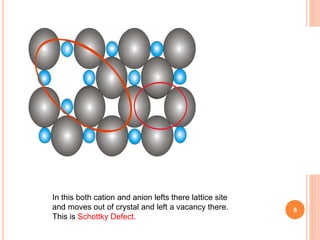
Frankel and Schottky defects occur in ionic crystals when ions leave their lattice sites. Frankel defects occur when an ion leaves its site and occupies an interstitial site, maintaining electrical neutrality and stoichiometry. Examples include AgCl and AgBr. Schottky defects occur when both cation and anion ions leave their lattice sites, creating vacancies that decrease the crystal's density. Examples include NaCl and KCl. The key difference between the defects is that Frankel defects do not change density while Schottky defects do due to vacancies formed by both ion types leaving lattice sites.