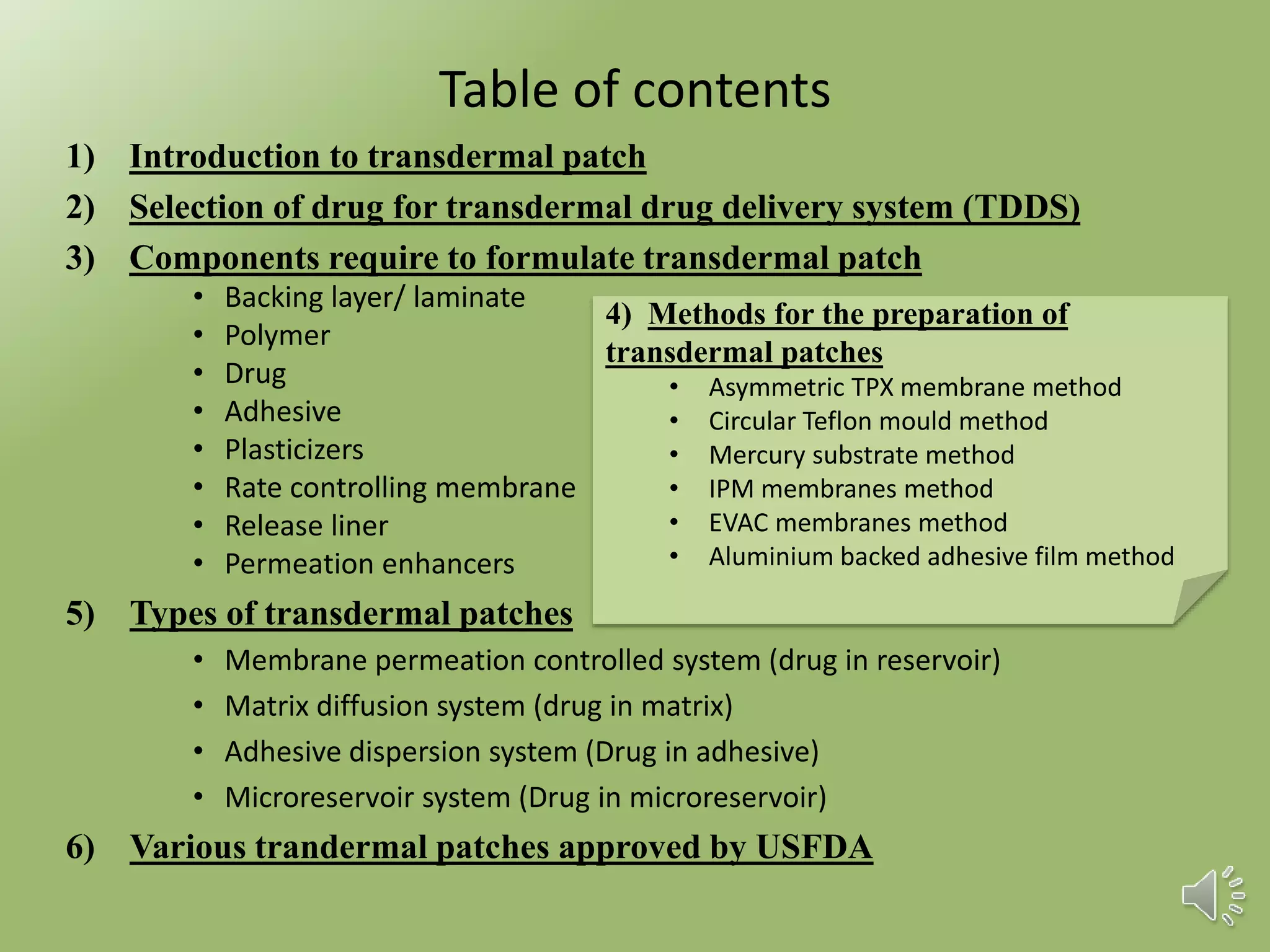
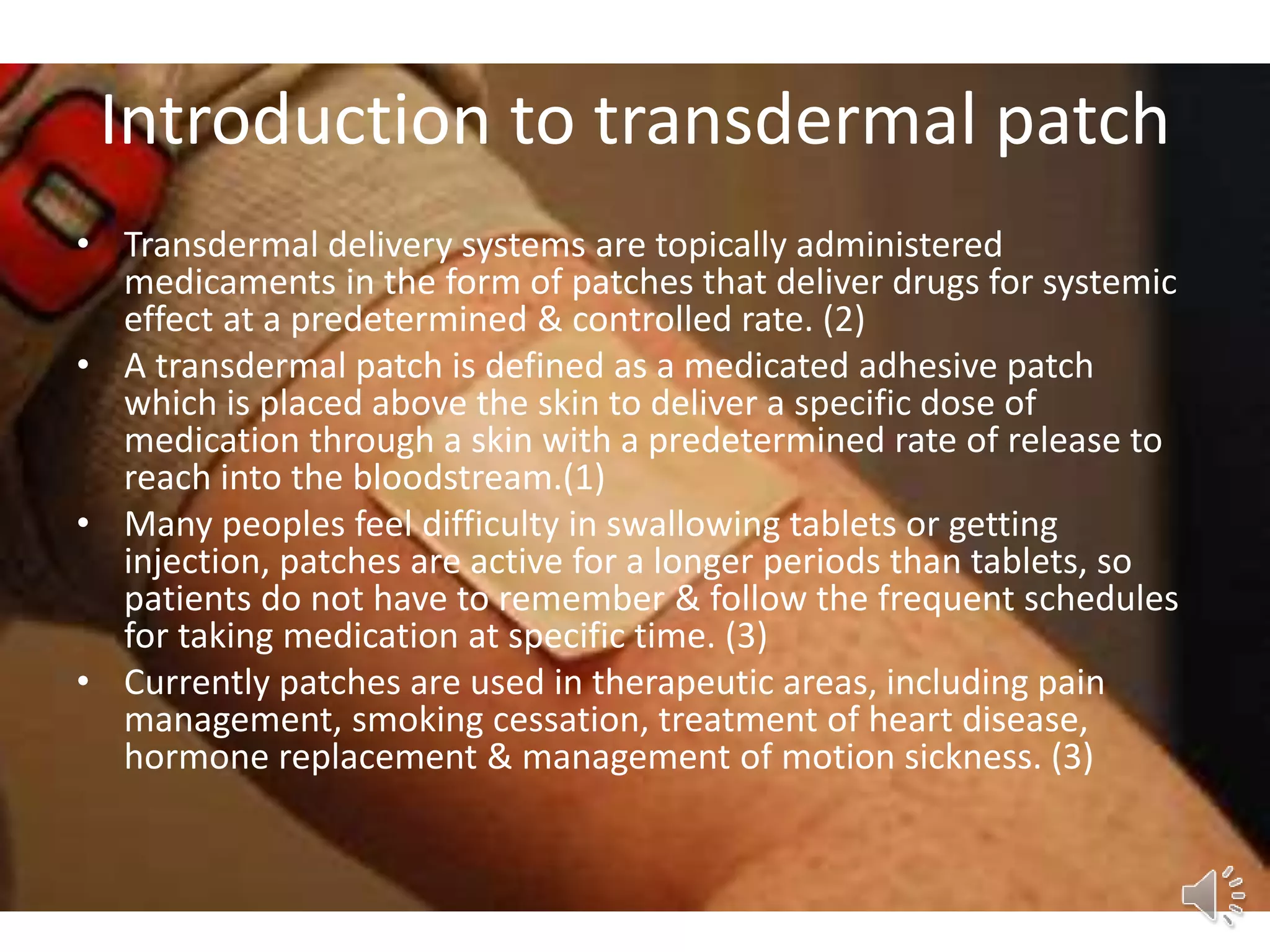
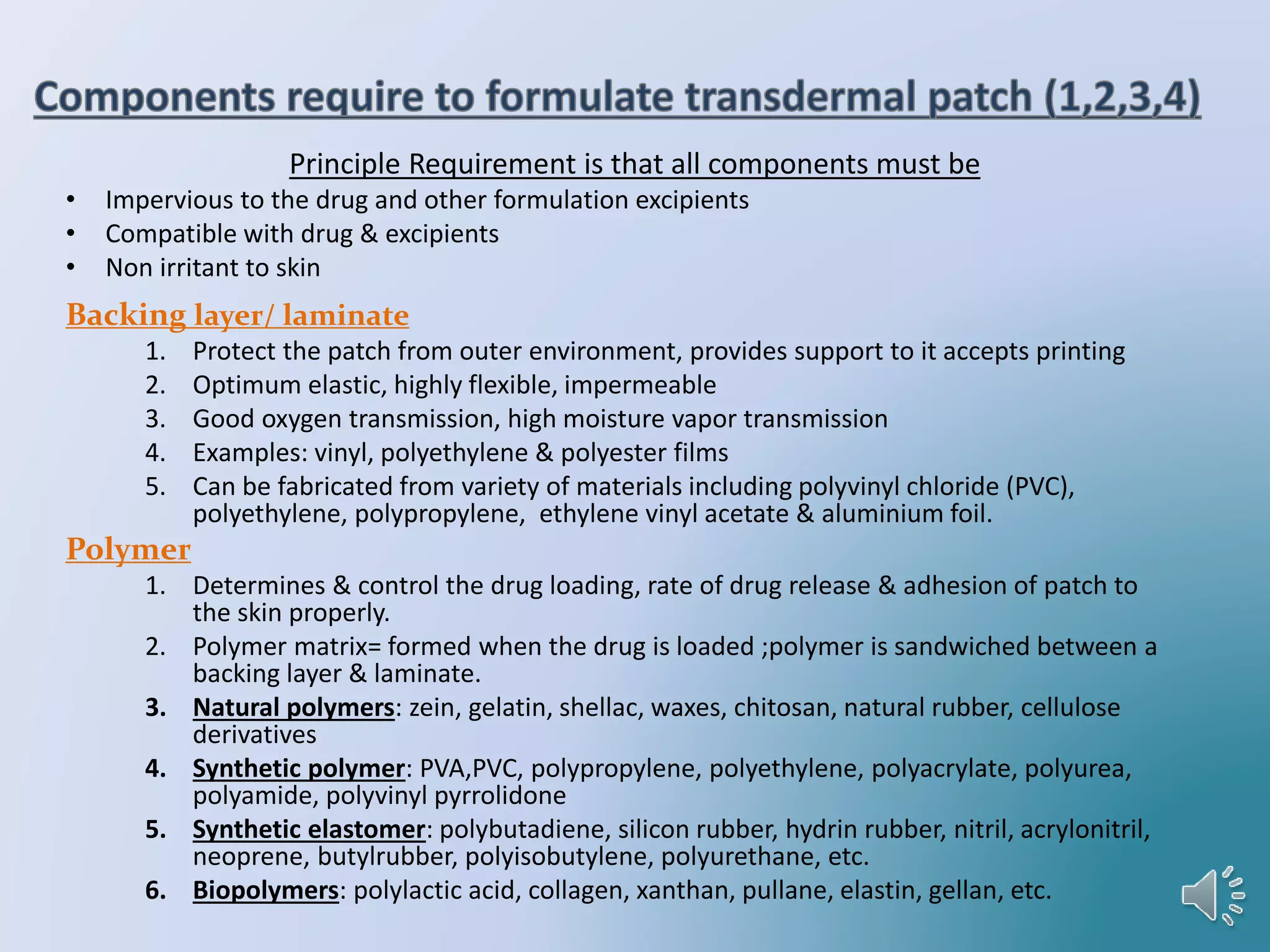
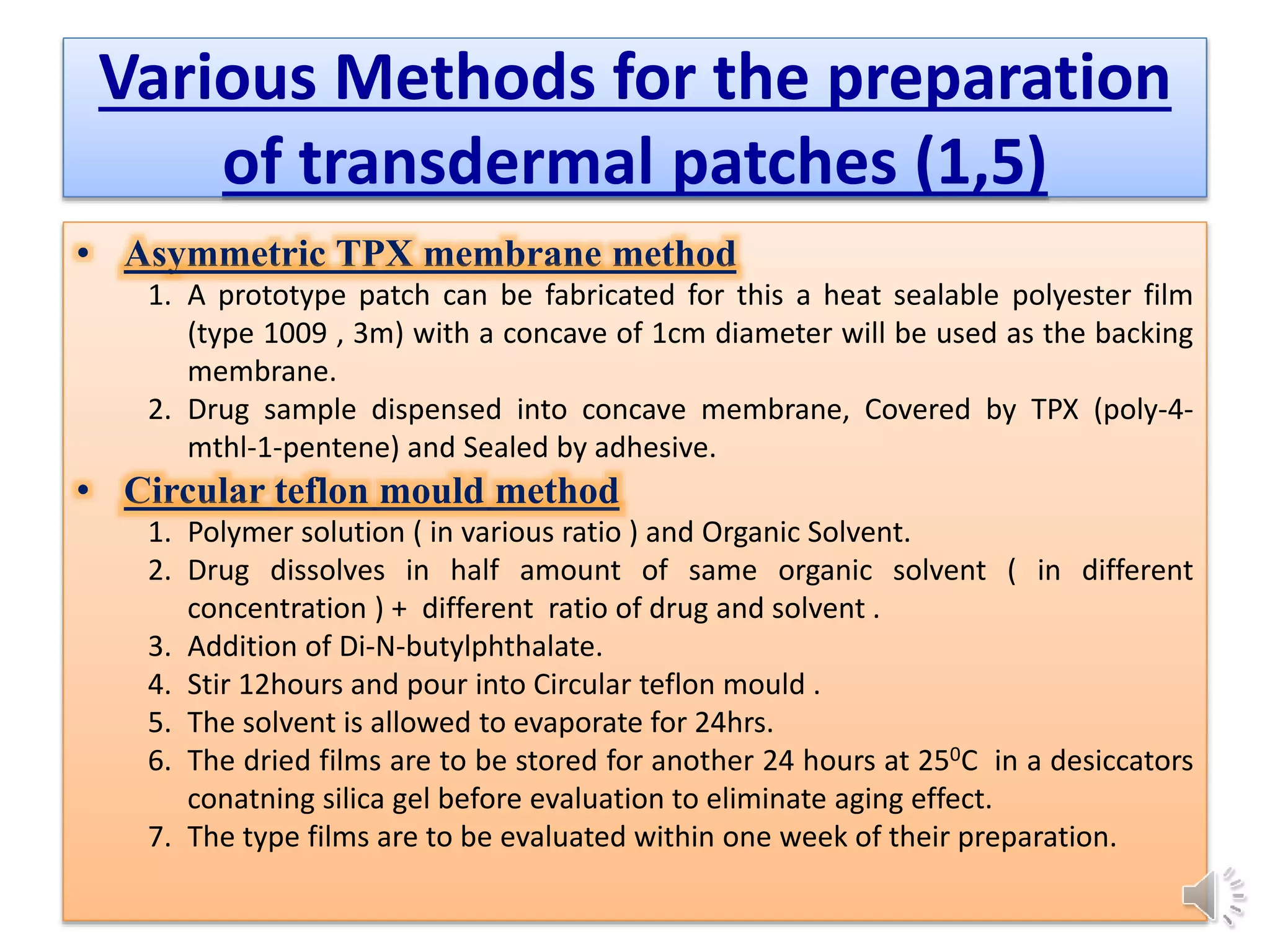
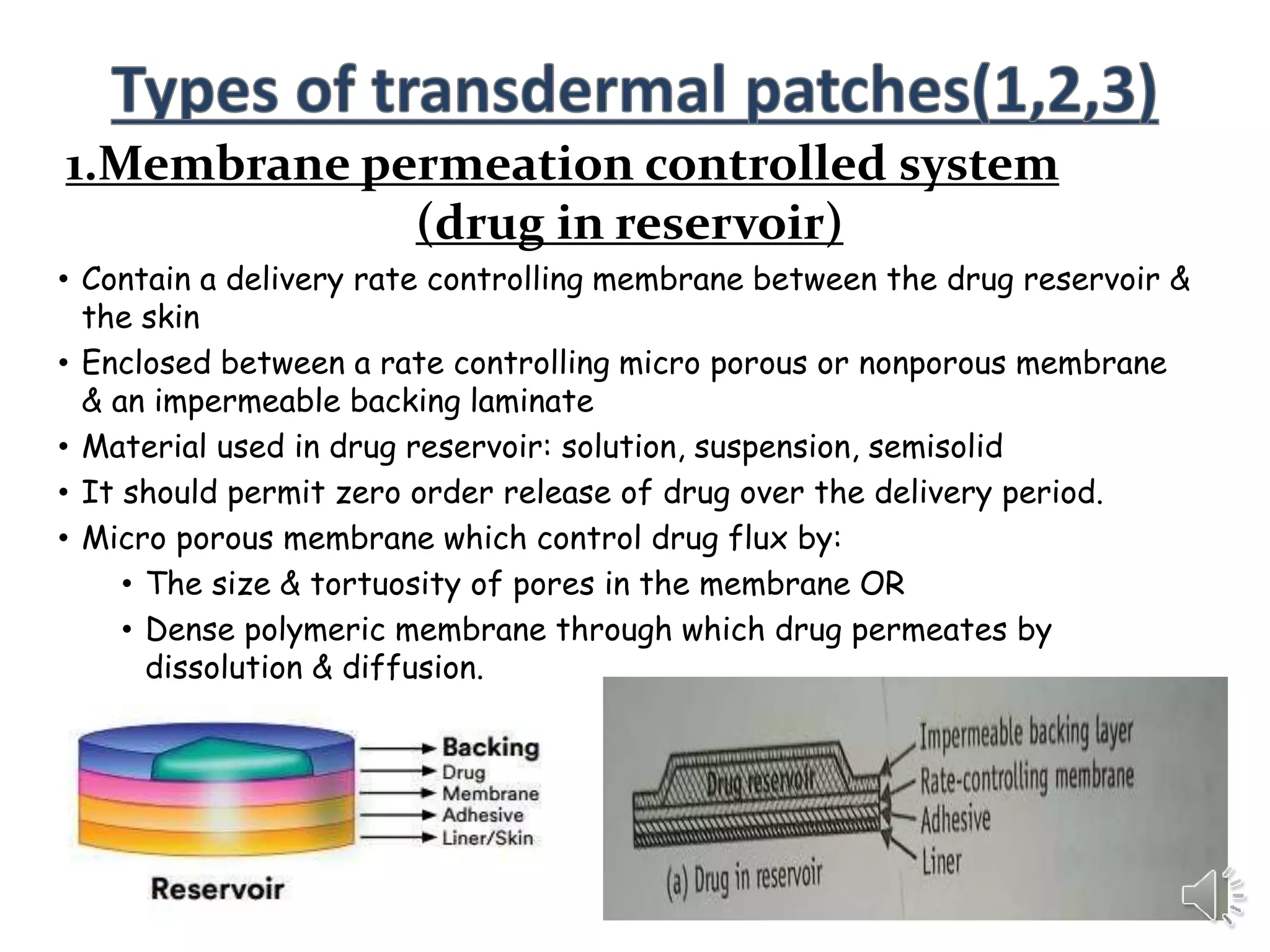
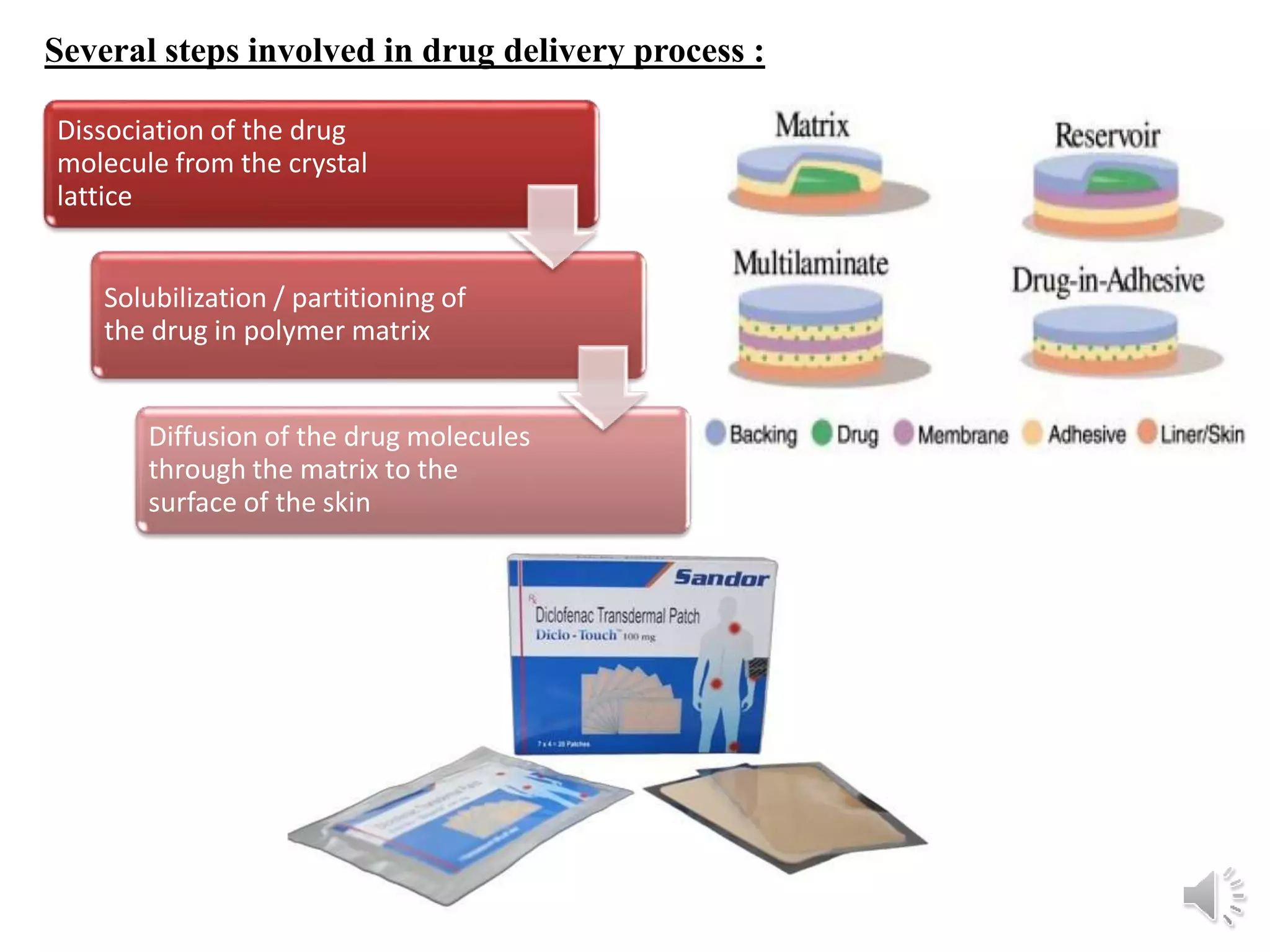
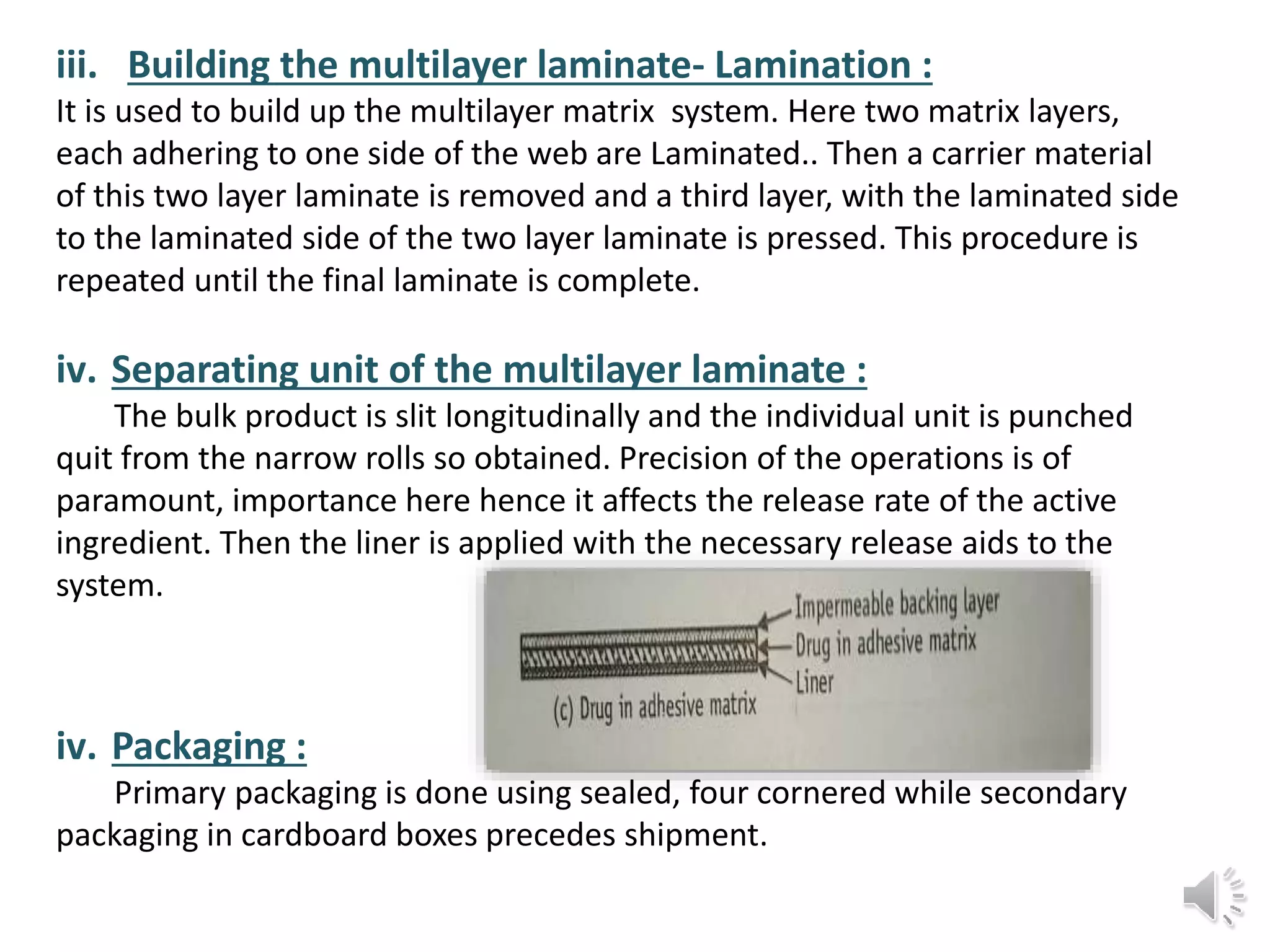
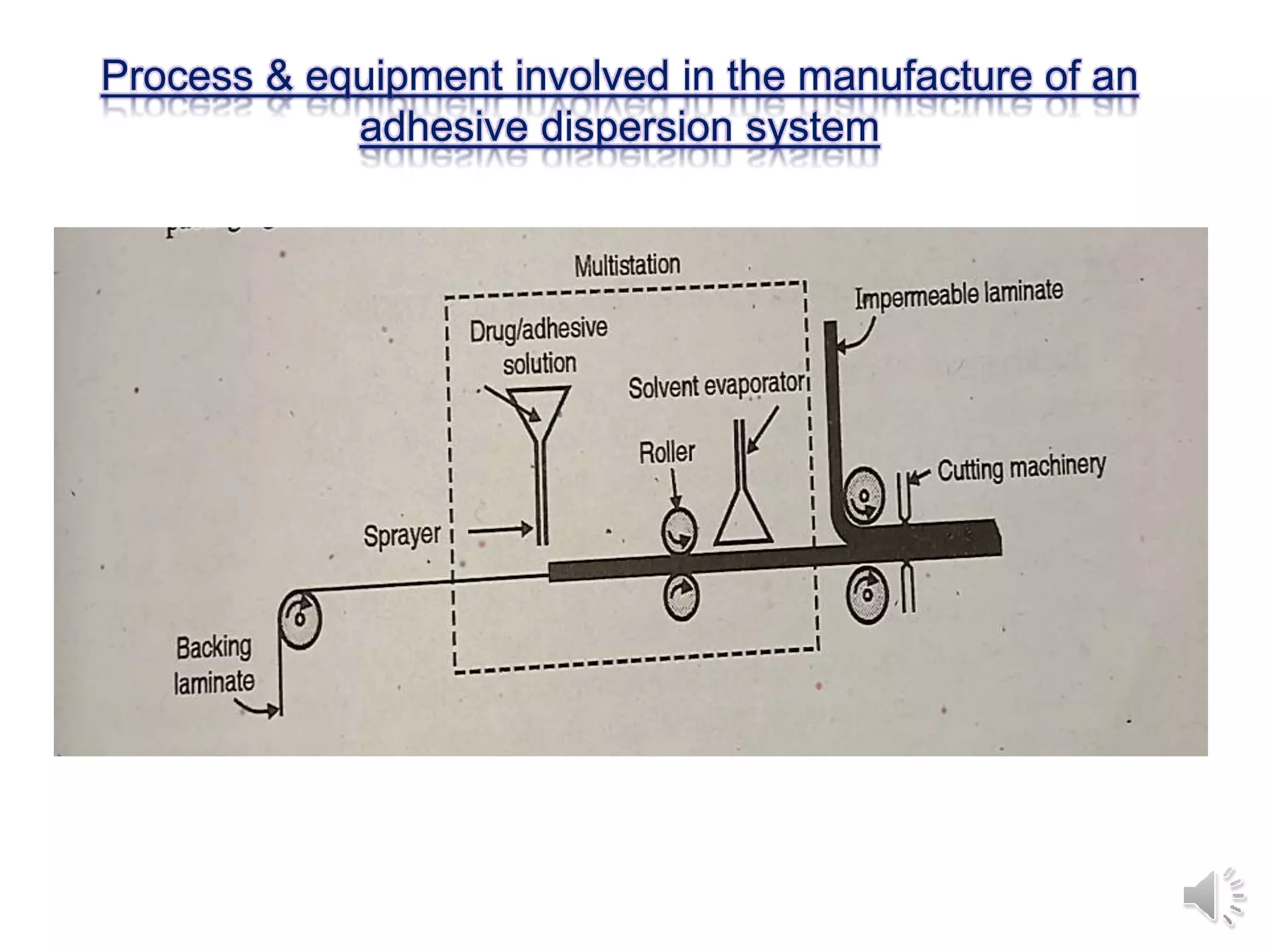
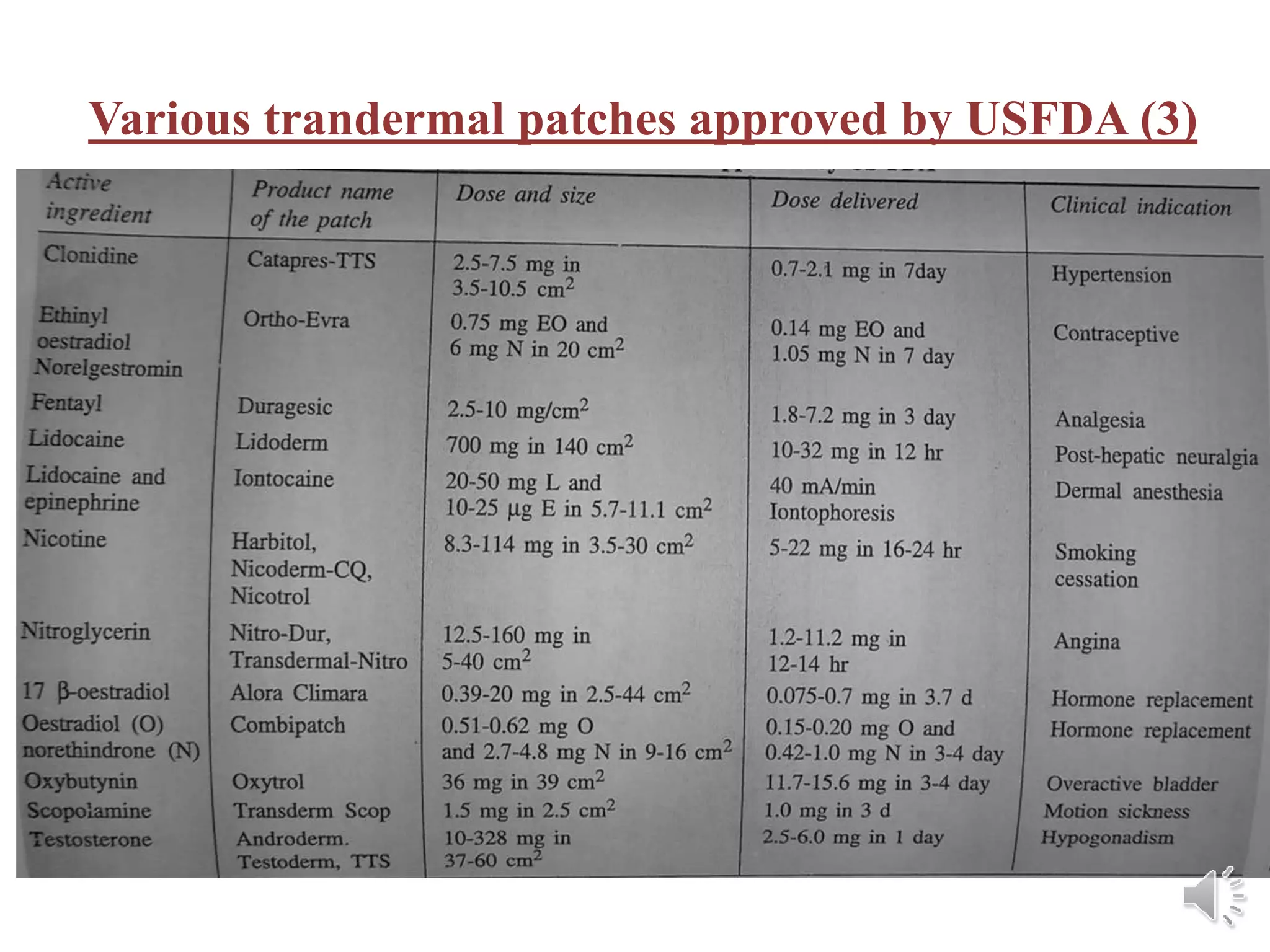
The document provides a comprehensive overview of transdermal drug delivery systems, detailing the components, types of patches, methods of formulation, and criteria for drug selection. It discusses the preparation of various transdermal patches, highlighting techniques like asymmetric tpx membrane and circular teflon mould methods, as well as the significance of materials such as polymers and adhesives. Additionally, it includes examples of transdermal patches approved by the US FDA and references relevant academic sources.