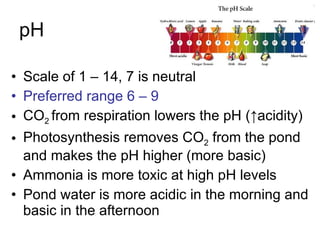
Fish have more efficient feed conversion ratios than land animals like cows and pigs due to their biology and environment. Fish feeds are more concentrated in nutrients than feeds for land animals. A balanced nutrition mix for fish includes protein, fat, carbohydrates, vitamins, and minerals. Proper storage, water quality factors like oxygen, temperature, pH, alkalinity, hardness, ammonia, nitrite, and salinity are important for fish health. Routine water testing and monitoring of these factors is necessary in aquaculture.