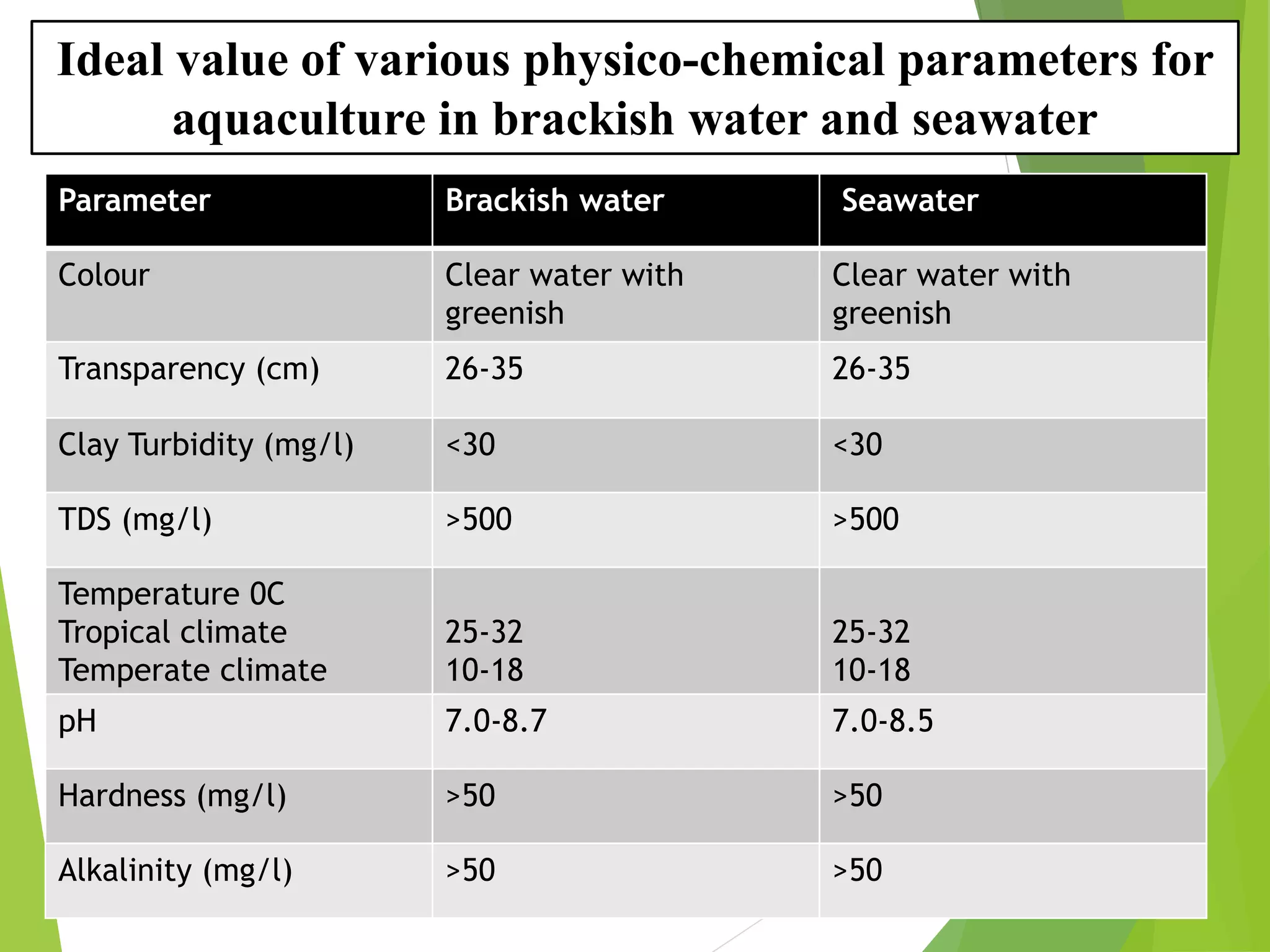
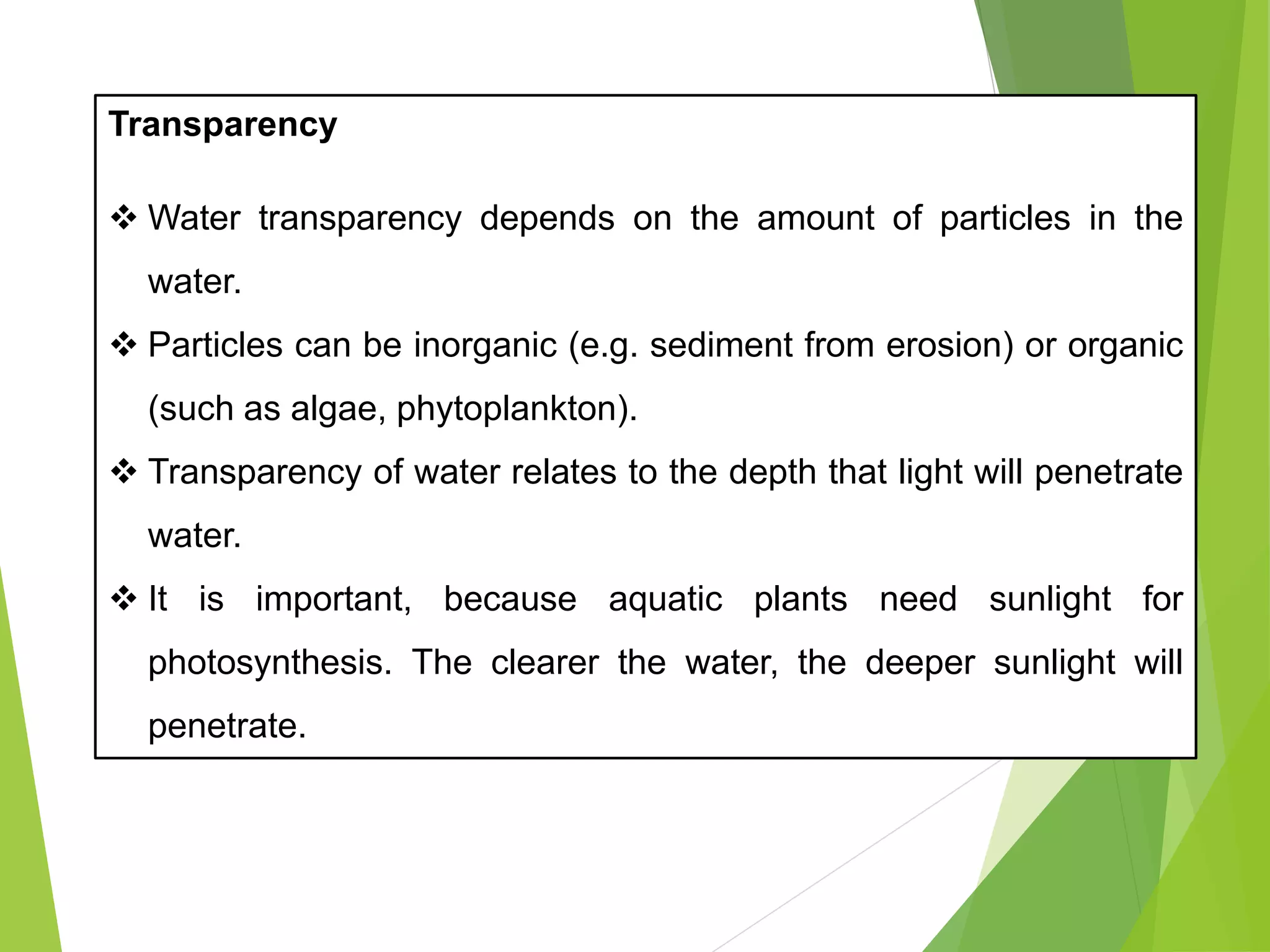
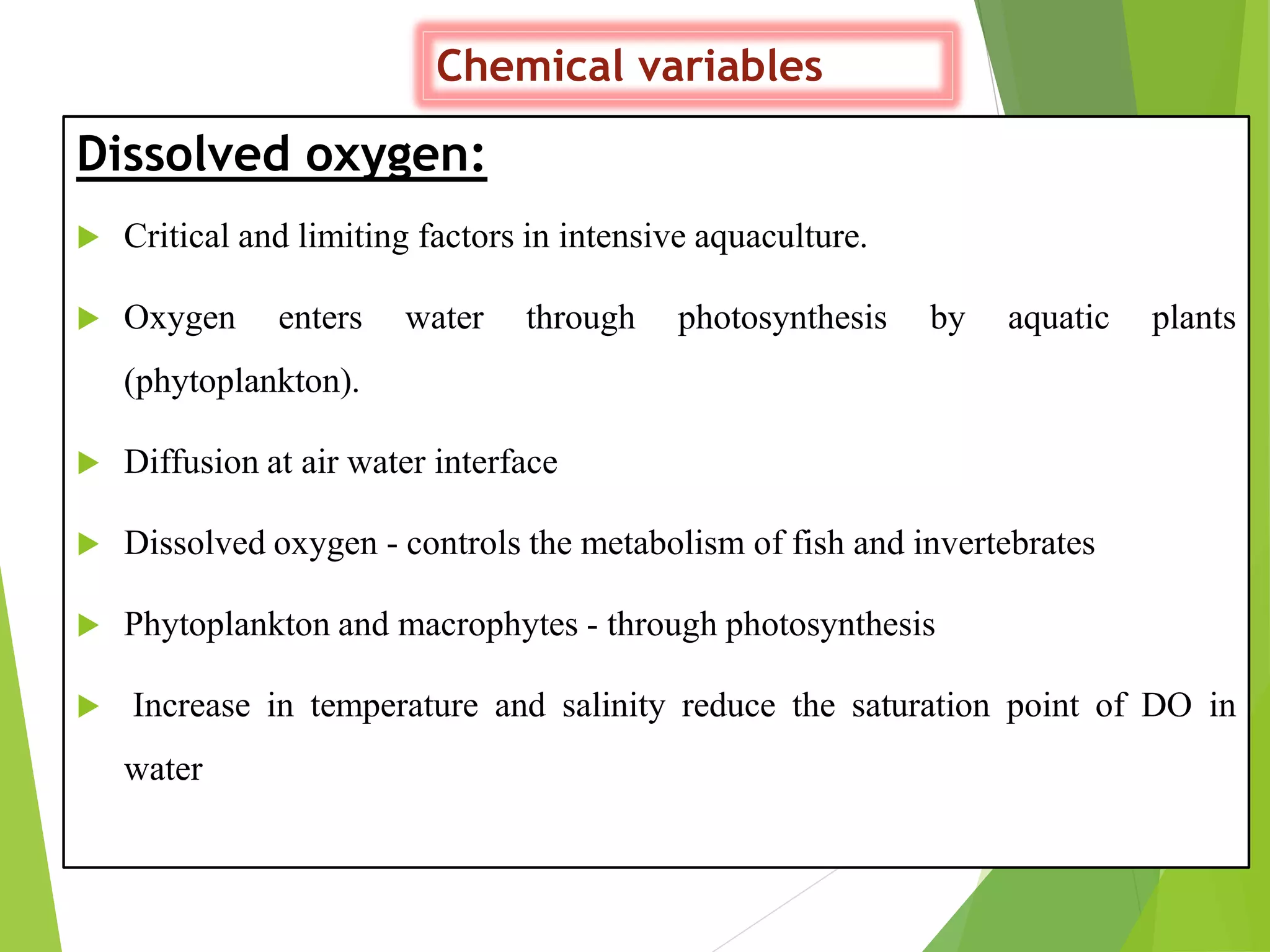
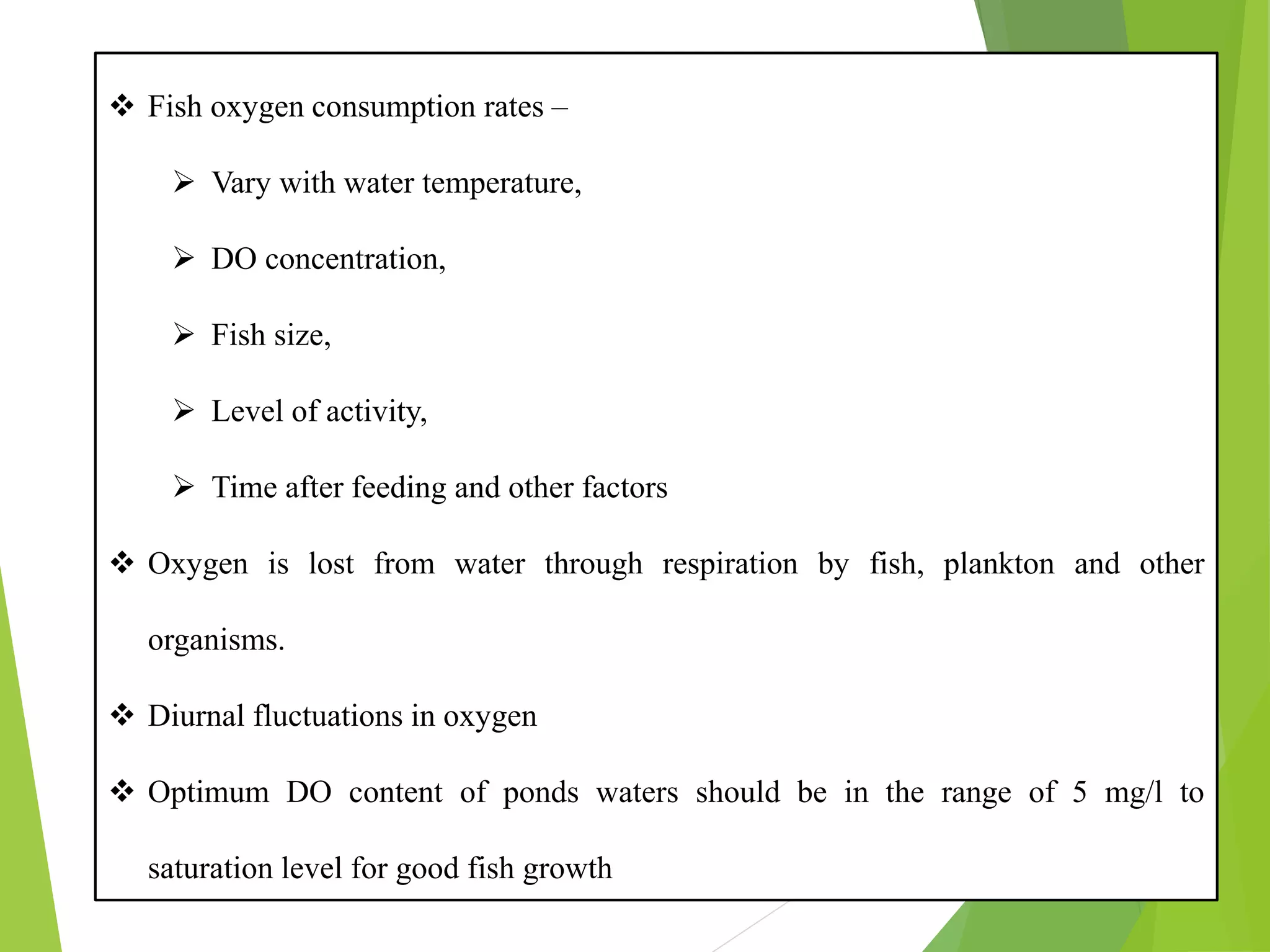
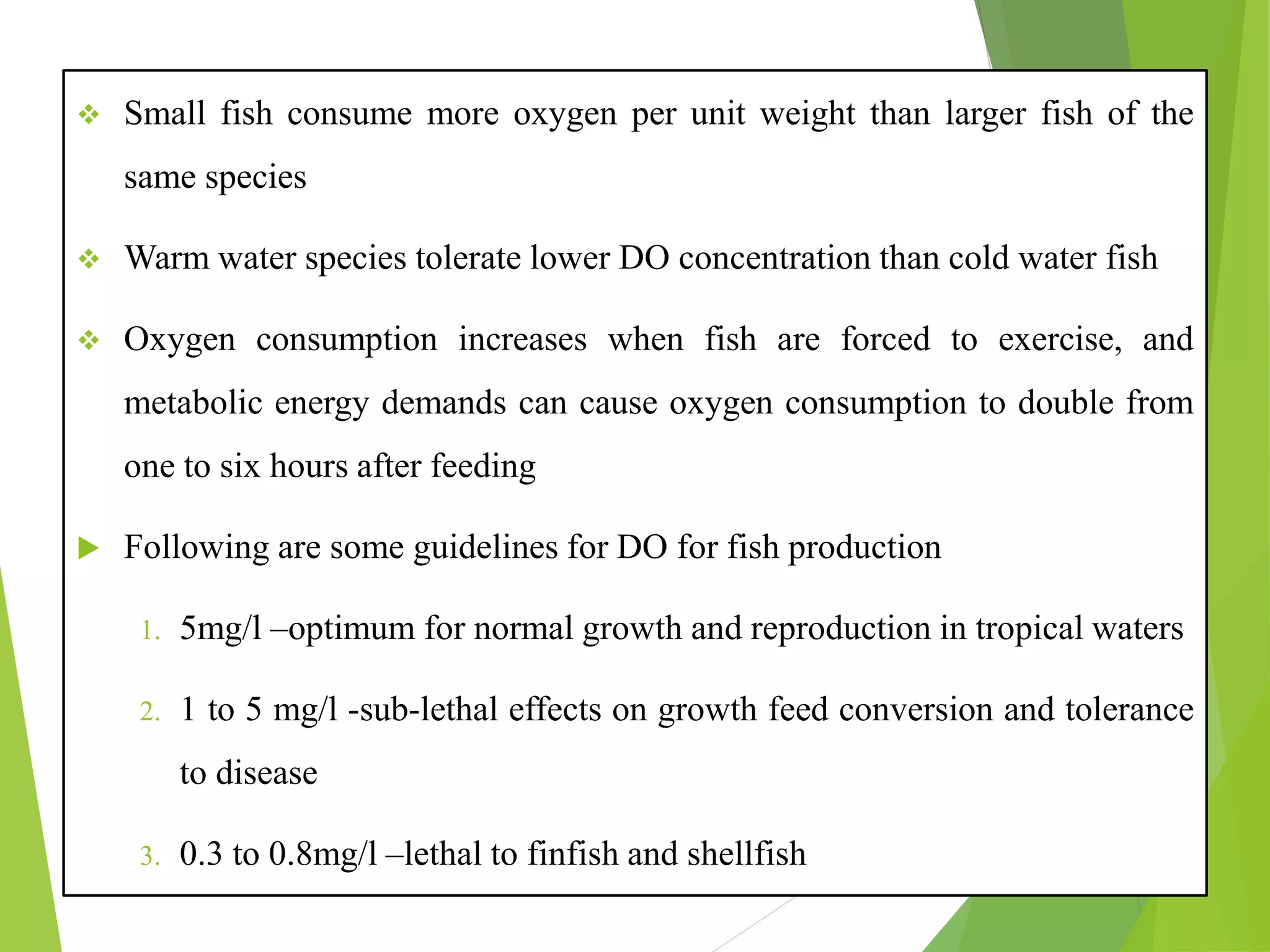
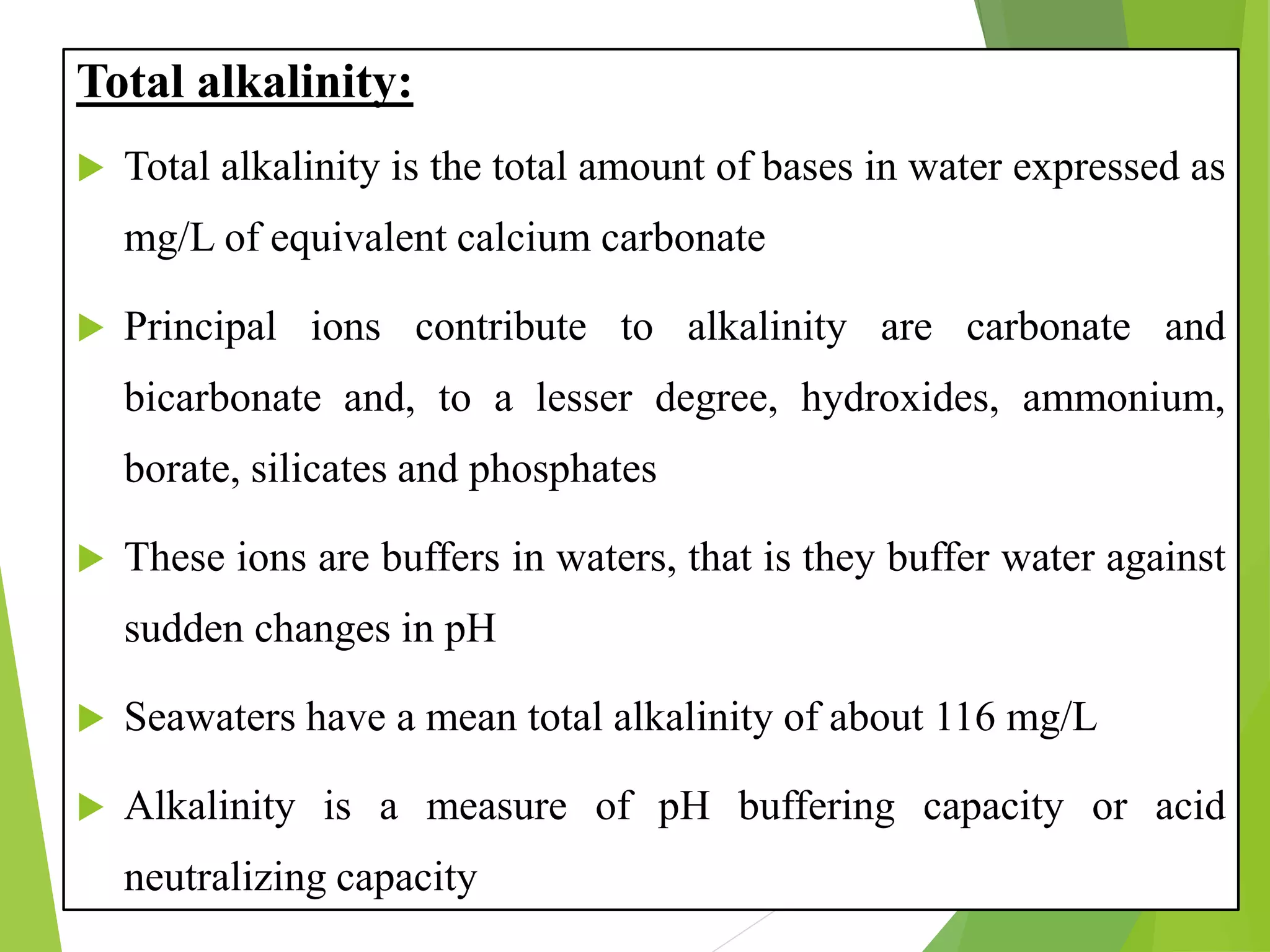
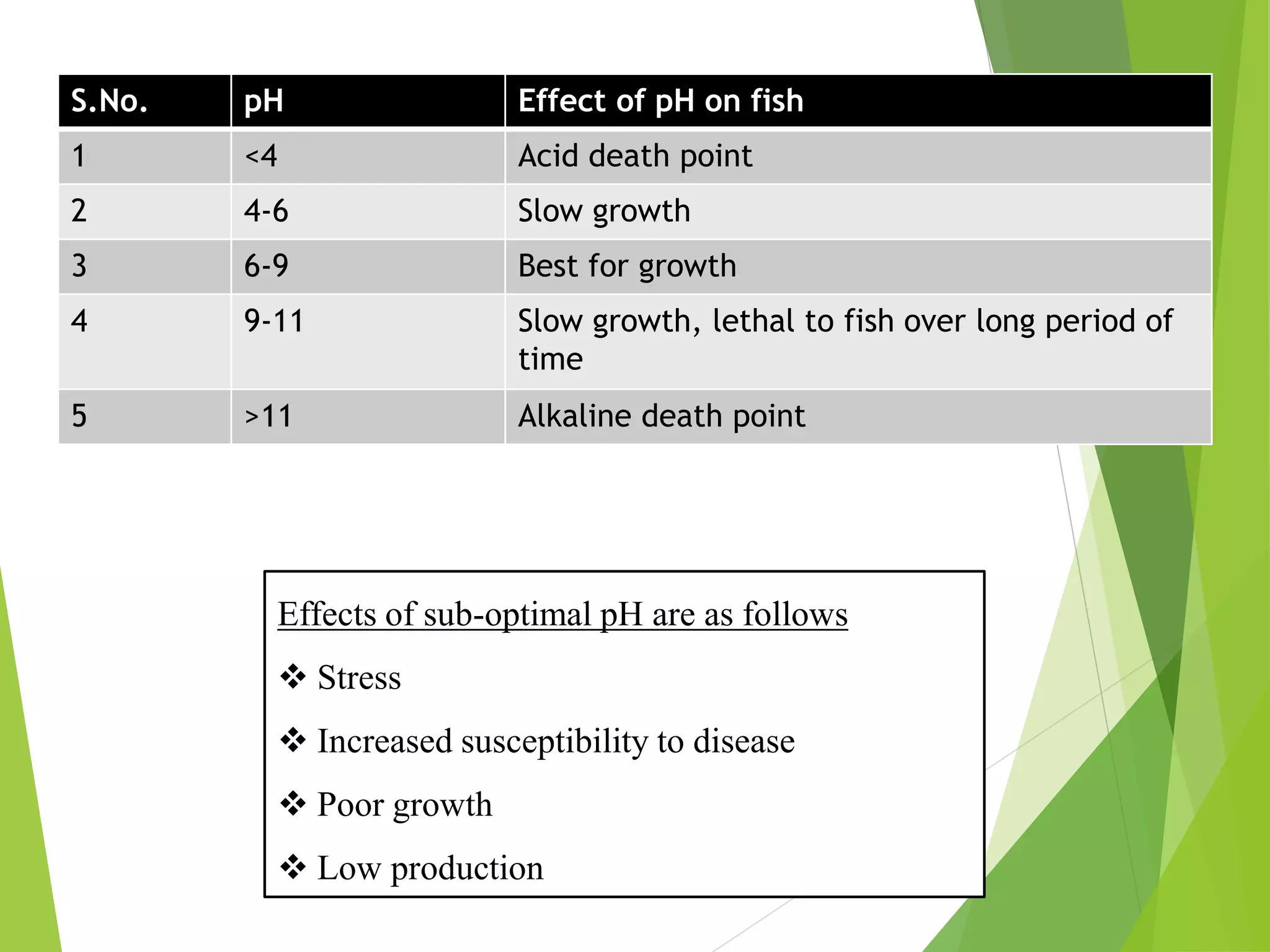
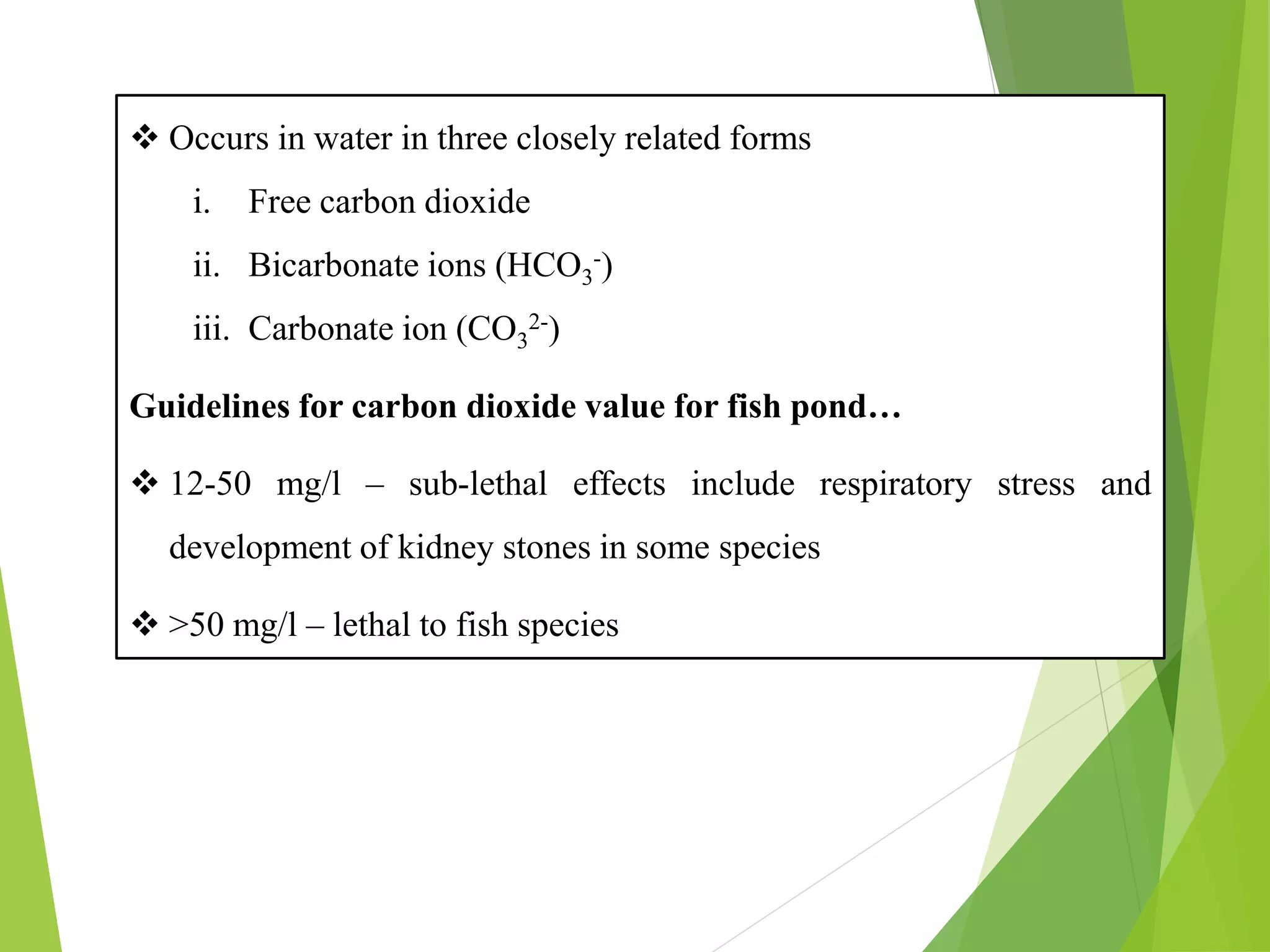
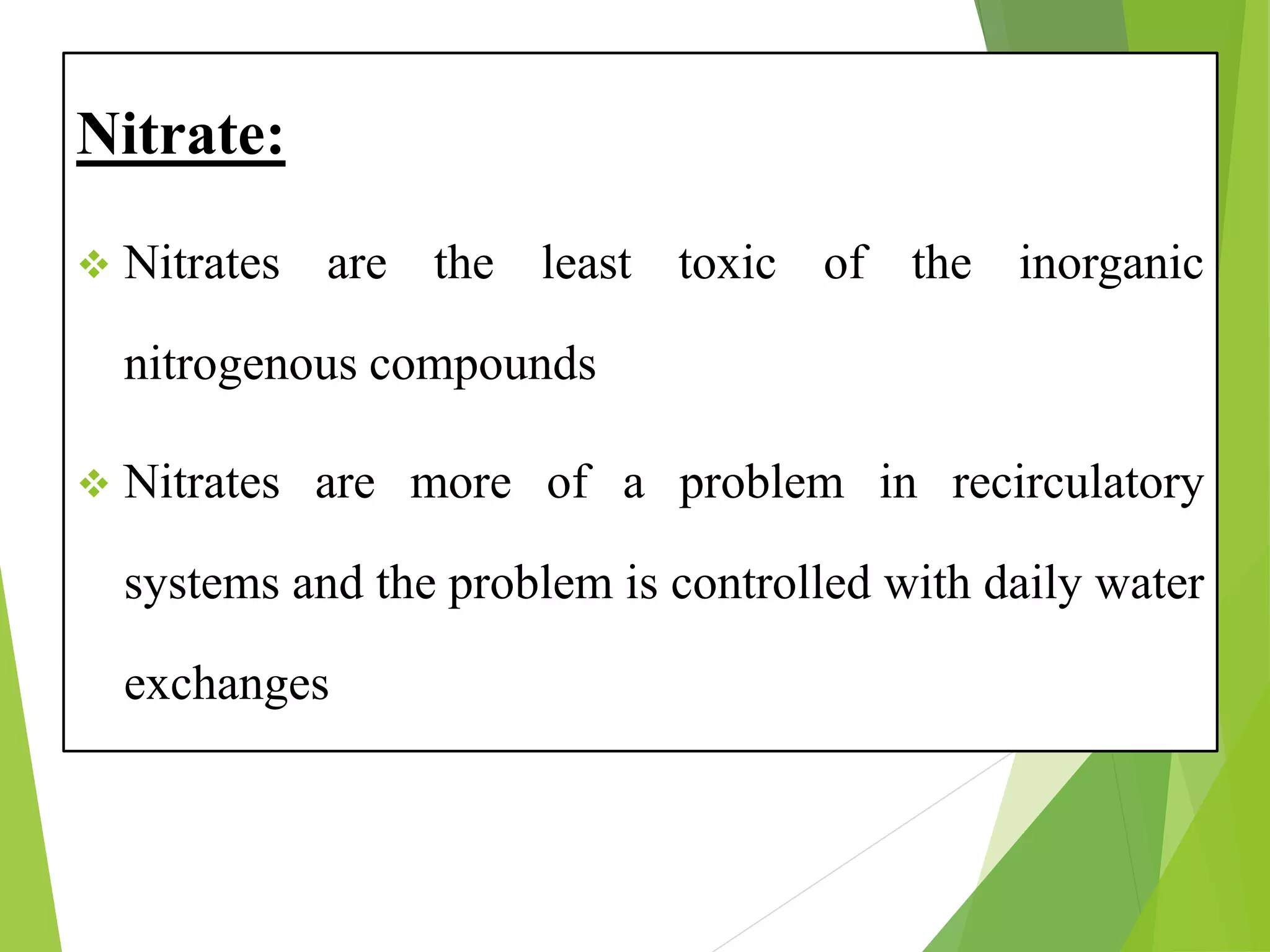
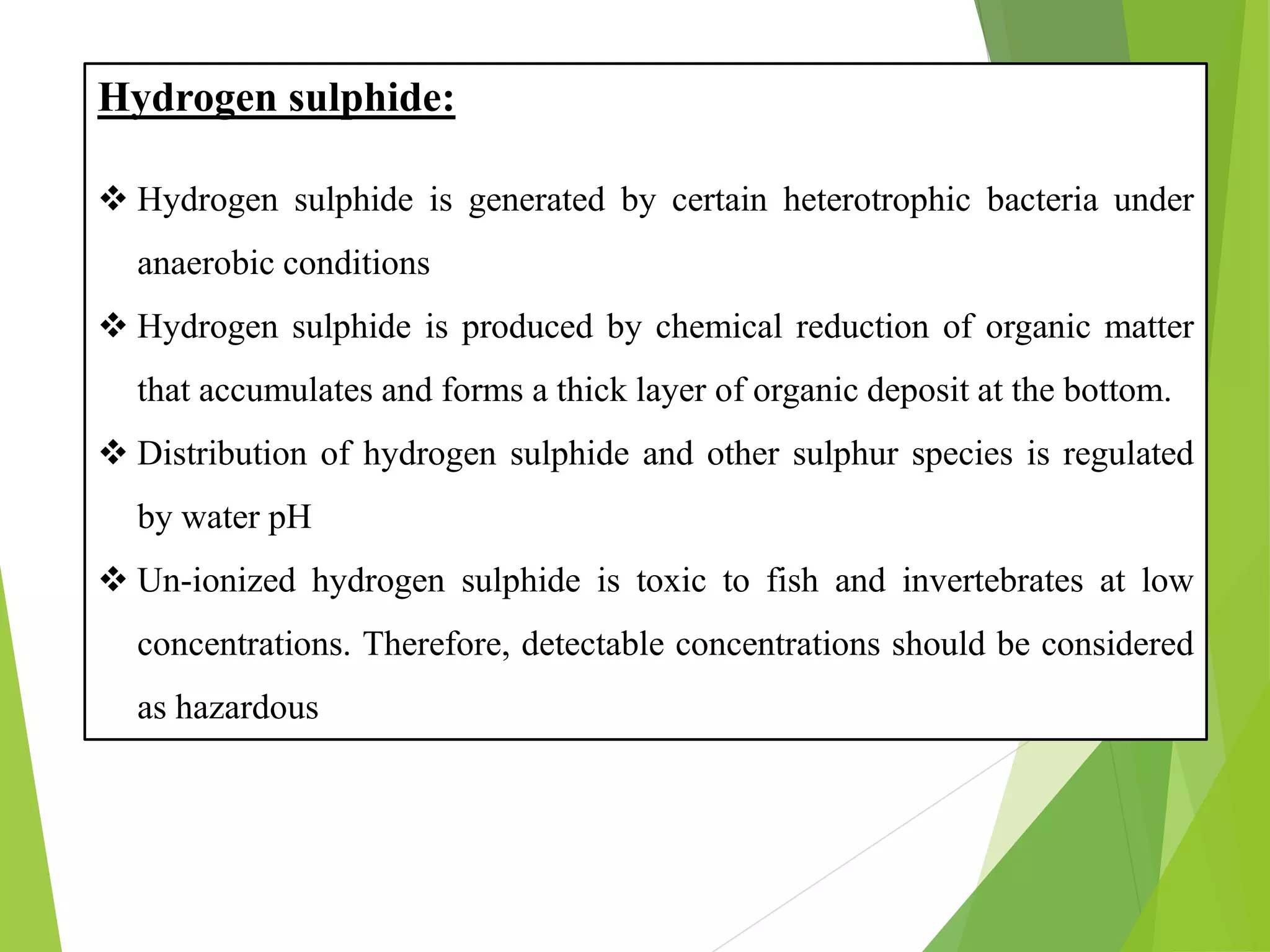
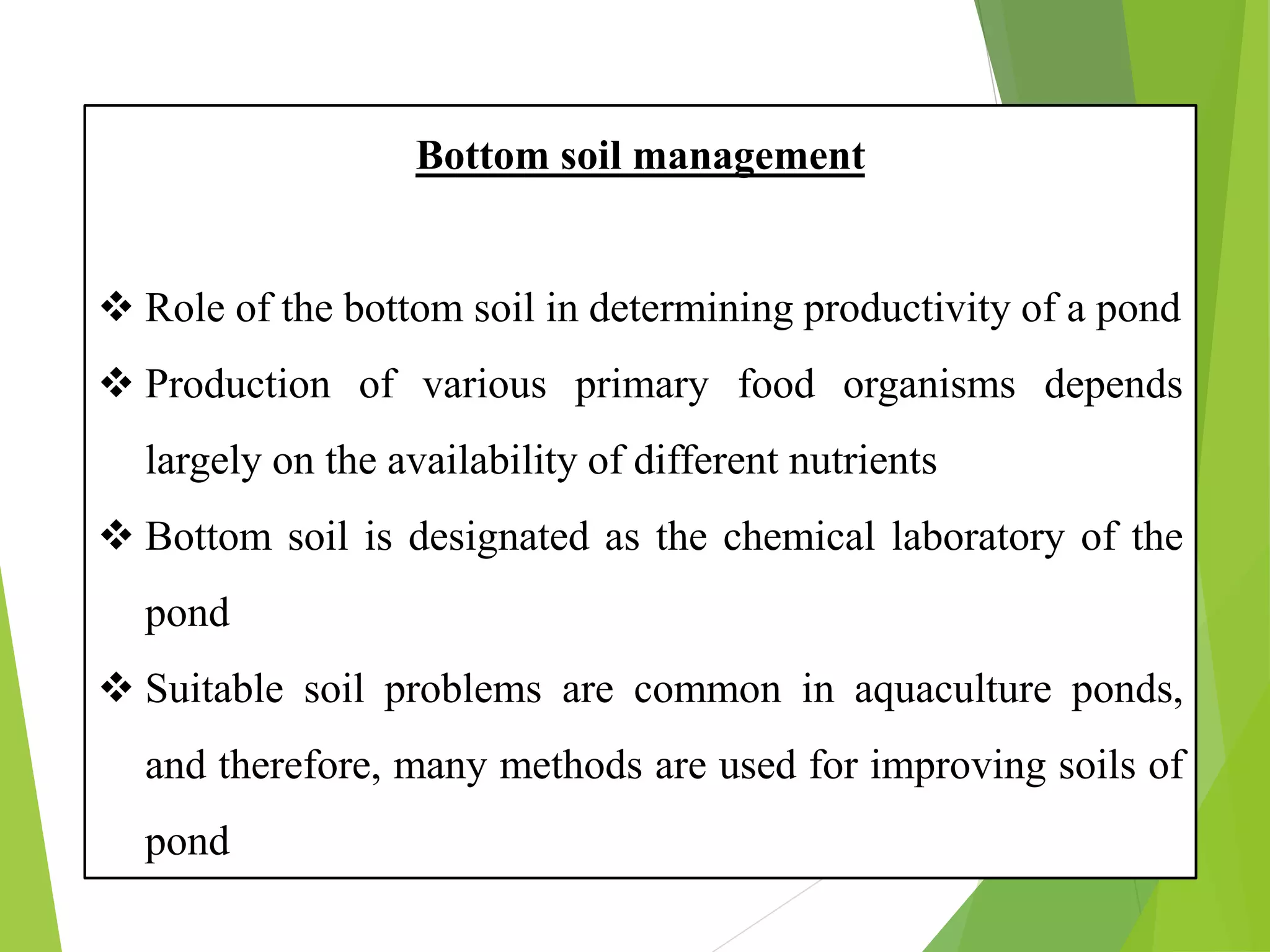
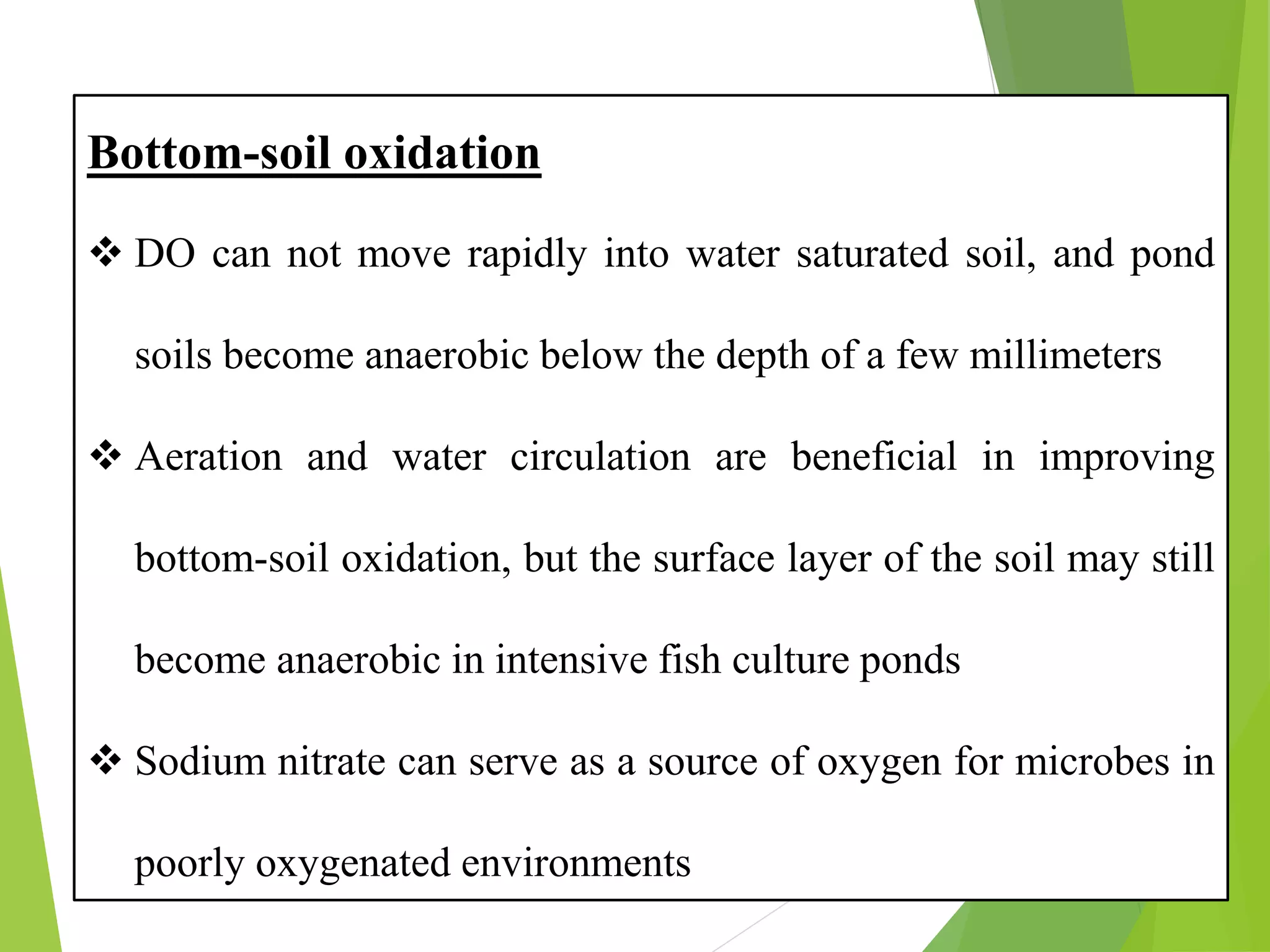
This document discusses water and soil quality management for aquaculture ponds and coastal systems. It provides ideal values for various physico-chemical parameters important for aquaculture, including temperature, salinity, dissolved oxygen, pH, alkalinity, hardness, and nitrogenous compounds. Maintaining optimal levels of these water quality parameters is essential for fish growth and health. The document also describes methods for monitoring parameters like turbidity, transparency and controlling imbalances in the system.