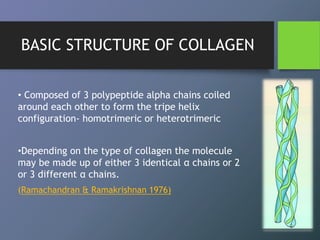
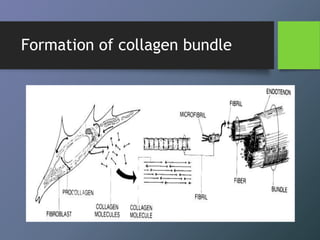
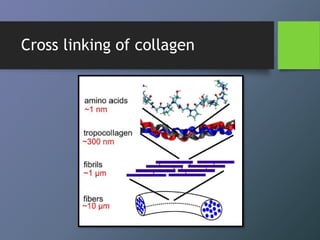
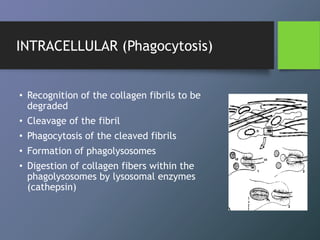
Collagen is the most abundant protein in mammals and forms the main fibrous component of connective tissues like skin, bone and cartilage. There are many types of collagen that vary in structure and function. Collagen provides strength, support and elasticity to tissues. It is synthesized through a complex process involving post-translational modifications. Collagen in the periodontium includes types I, III, IV, V, VI and XII which provide structure and allow for adaptation to forces. The periodontal ligament has the most rapid collagen turnover of tissues in the body to enable response to occlusion forces.



![COMPONENTS OF EXTRACELLULAR
MATRIX
PROTEOGLYCANS
• (Mucoproteins) :
Conjugated proteins
• Protein +
Carbohydrate
• Carbohydrate part is
in the form of
Glycosaminoglycans
[GAGs].
• Versican
• Decorin
• Biglycan
• Syndecan
NON COLLAGENOUS
PROTEINS
• Elastin
• Fibronectin
• Laminin
• Osteocalcin
• Osteopontin
• Bone sialoprotein
• Osteonectin
• Tenascin
STRUCTURAL PROTEINS
• Collagen
• Elastin
• Keratin (epidermal
tissues)](https://image.slidesharecdn.com/collagenppt-151201072026-lva1-app6892/85/Collagen-4-320.jpg)