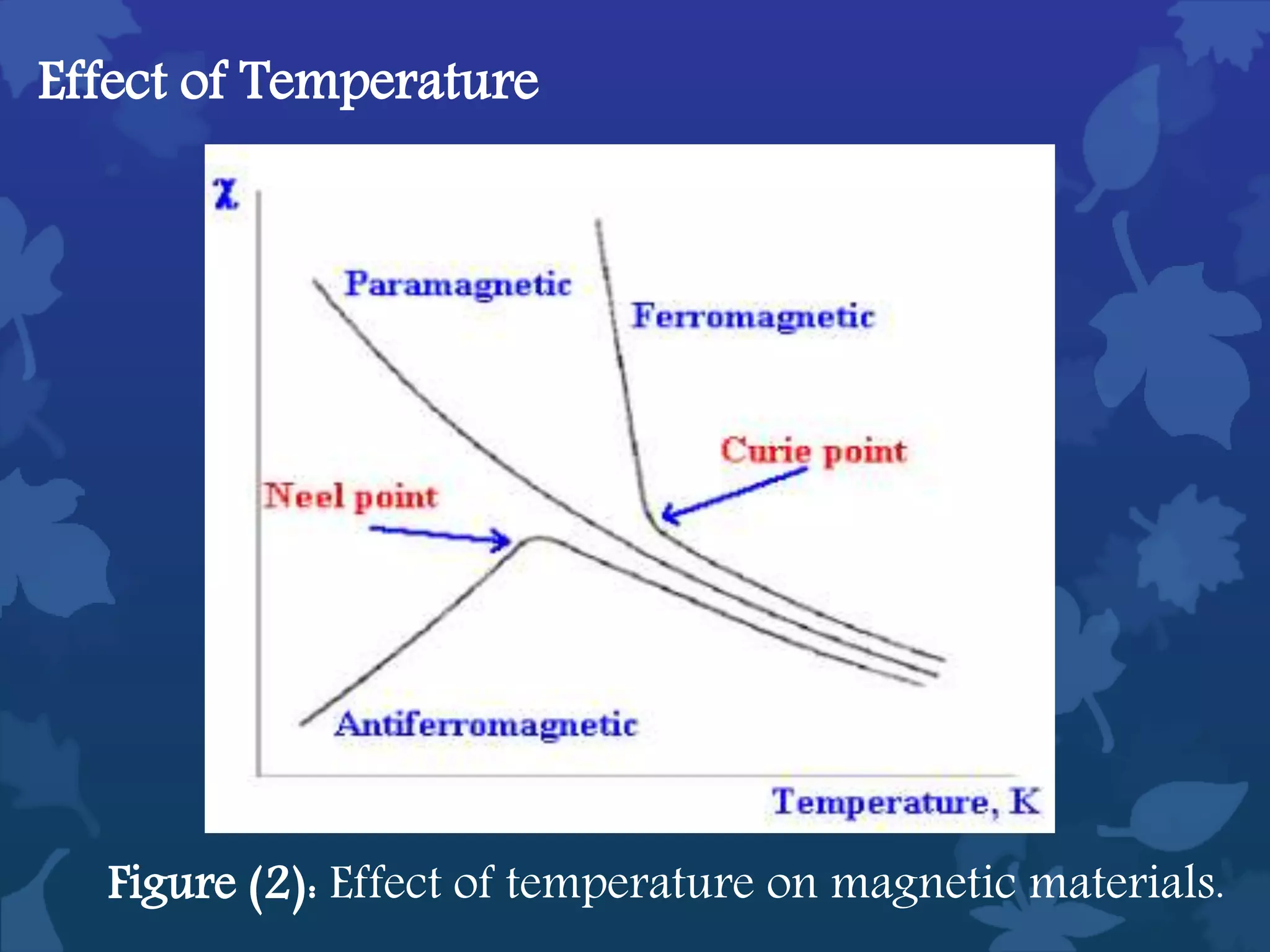
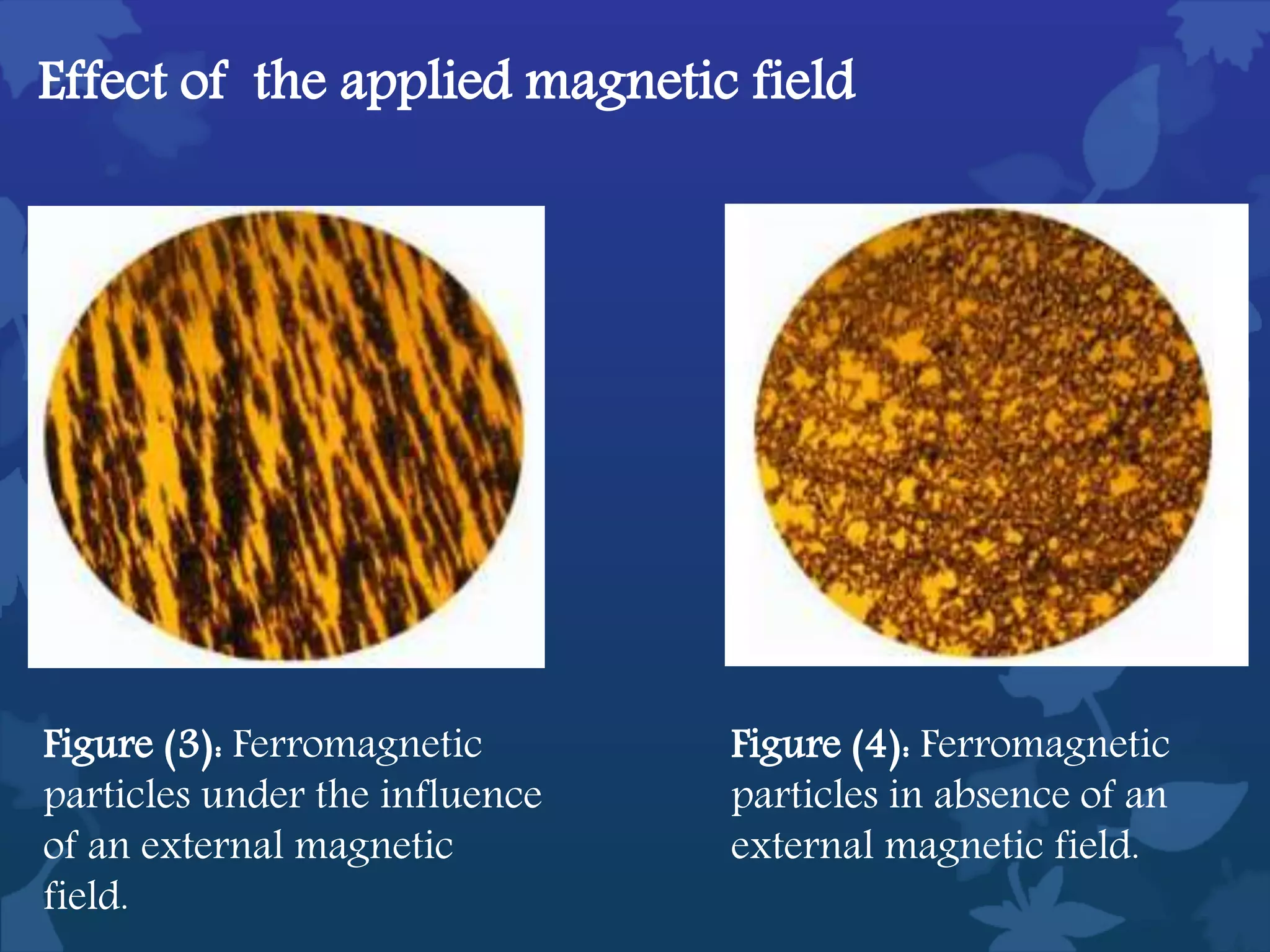
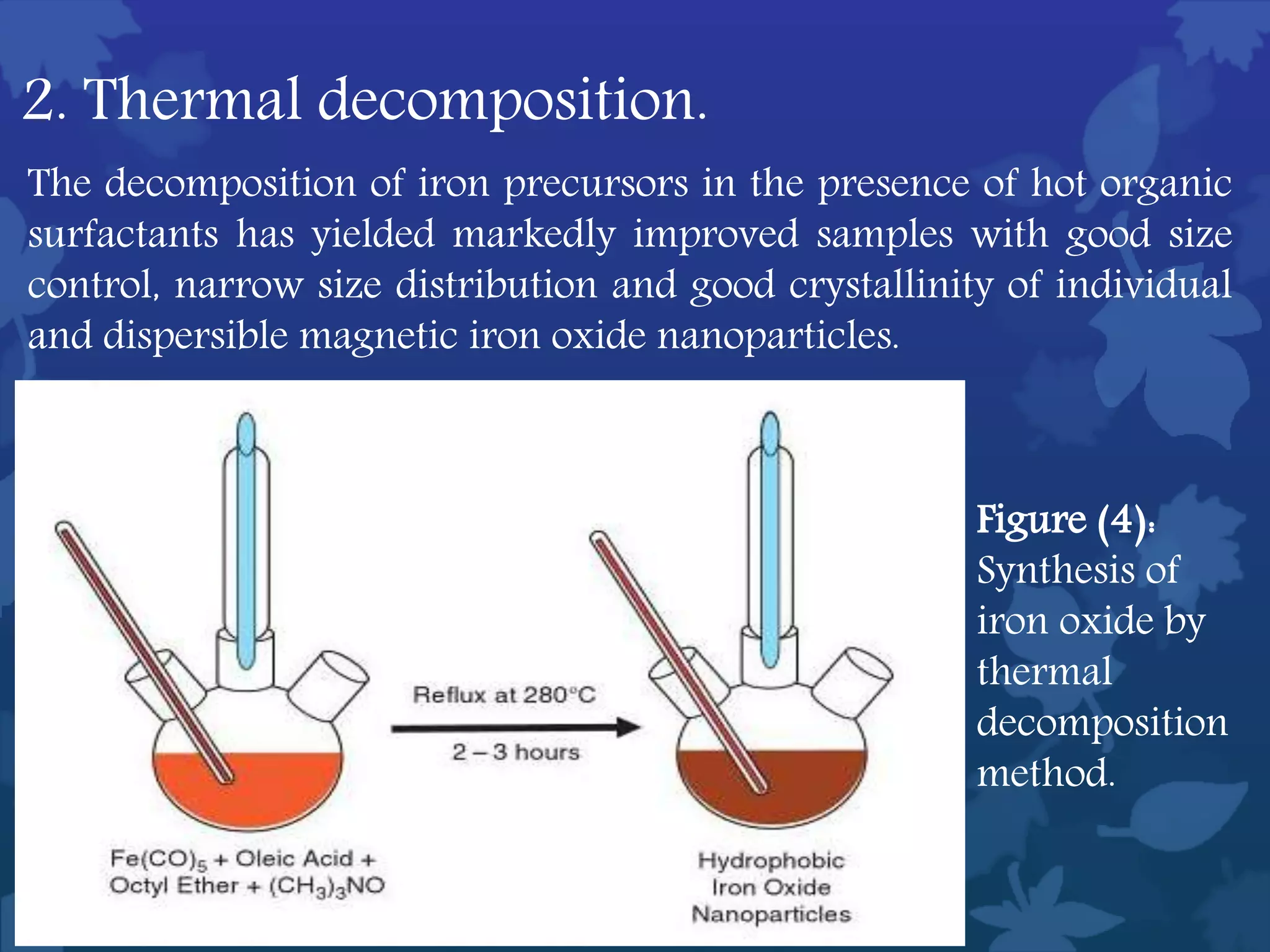
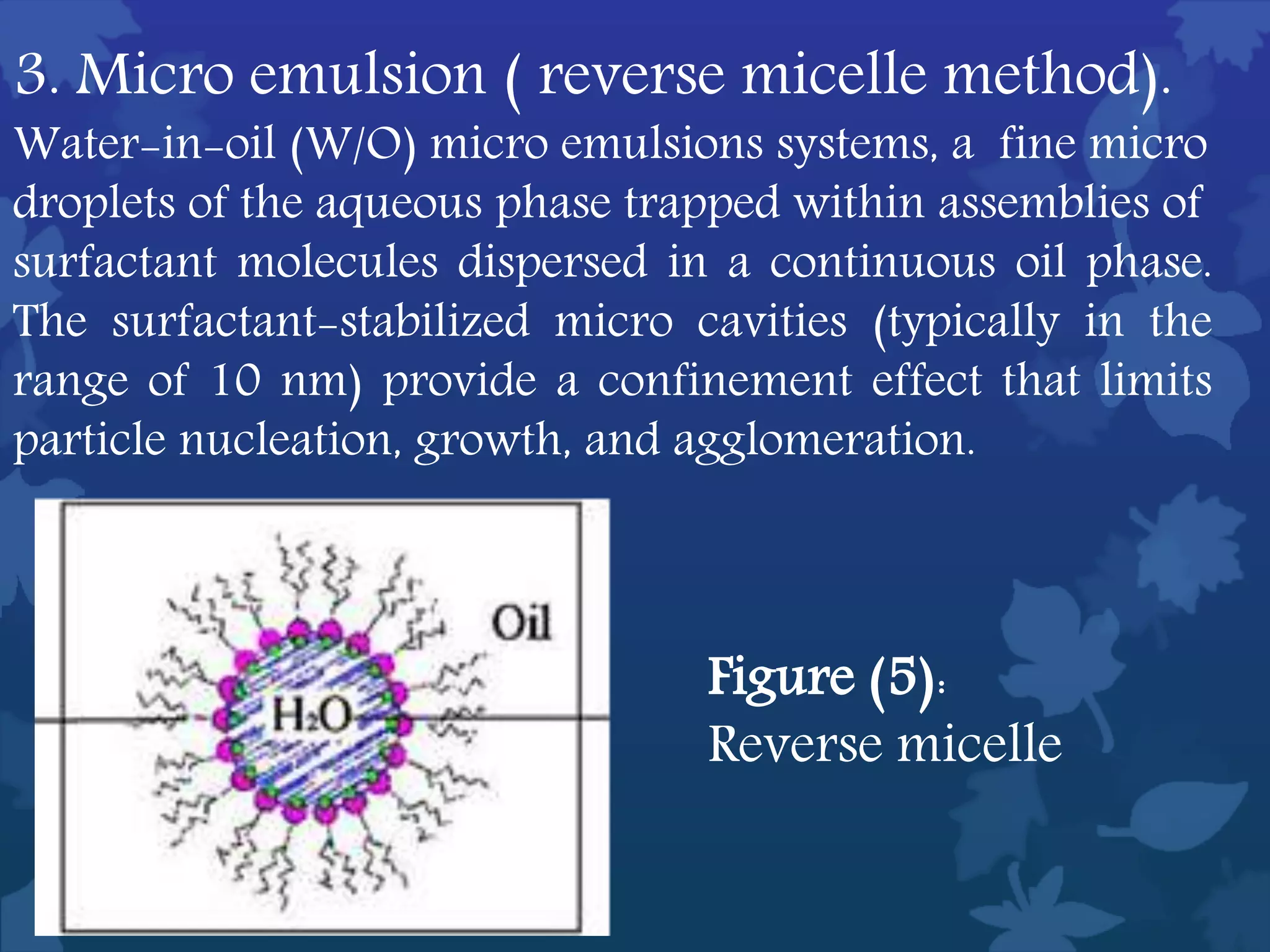
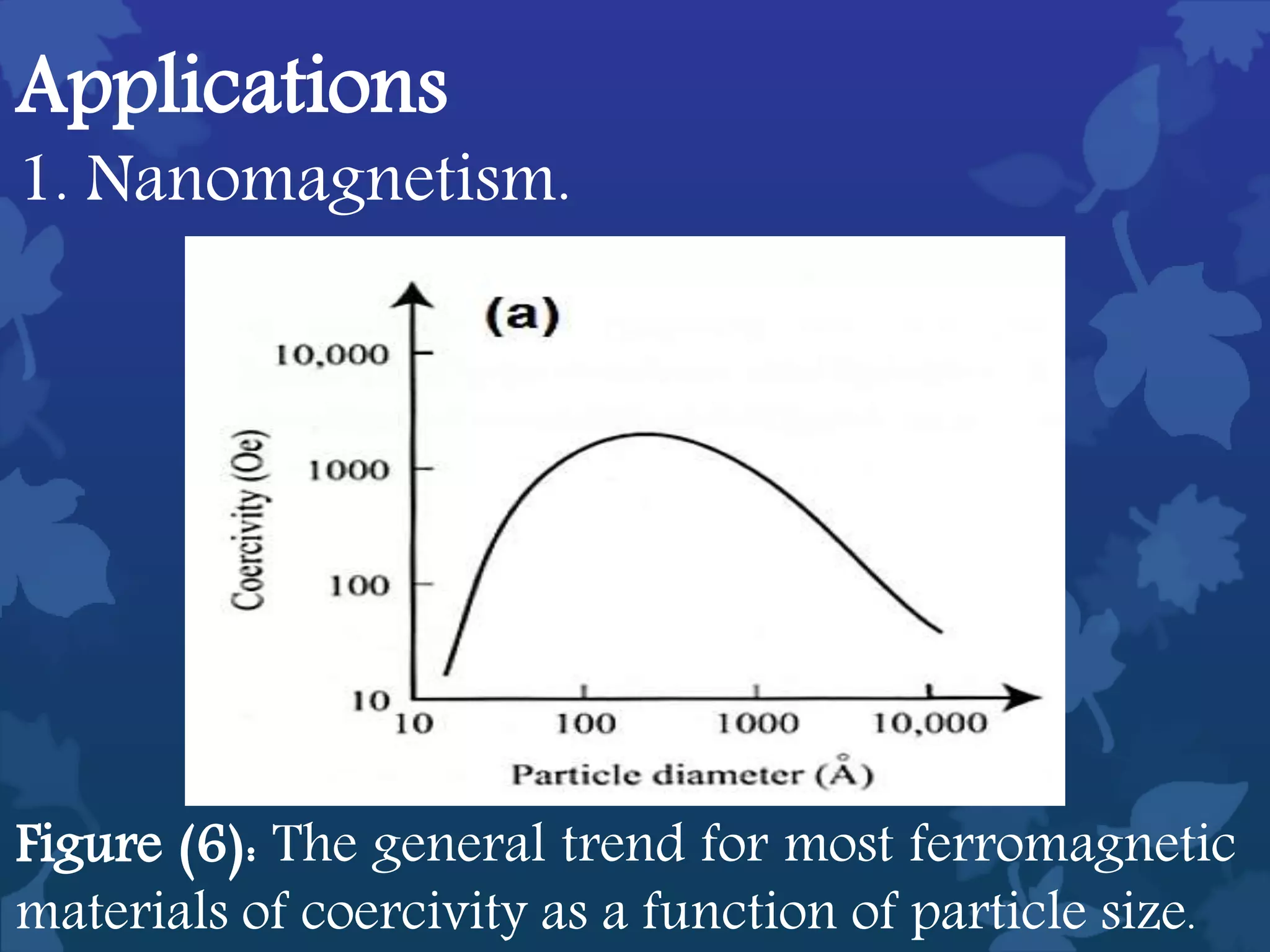
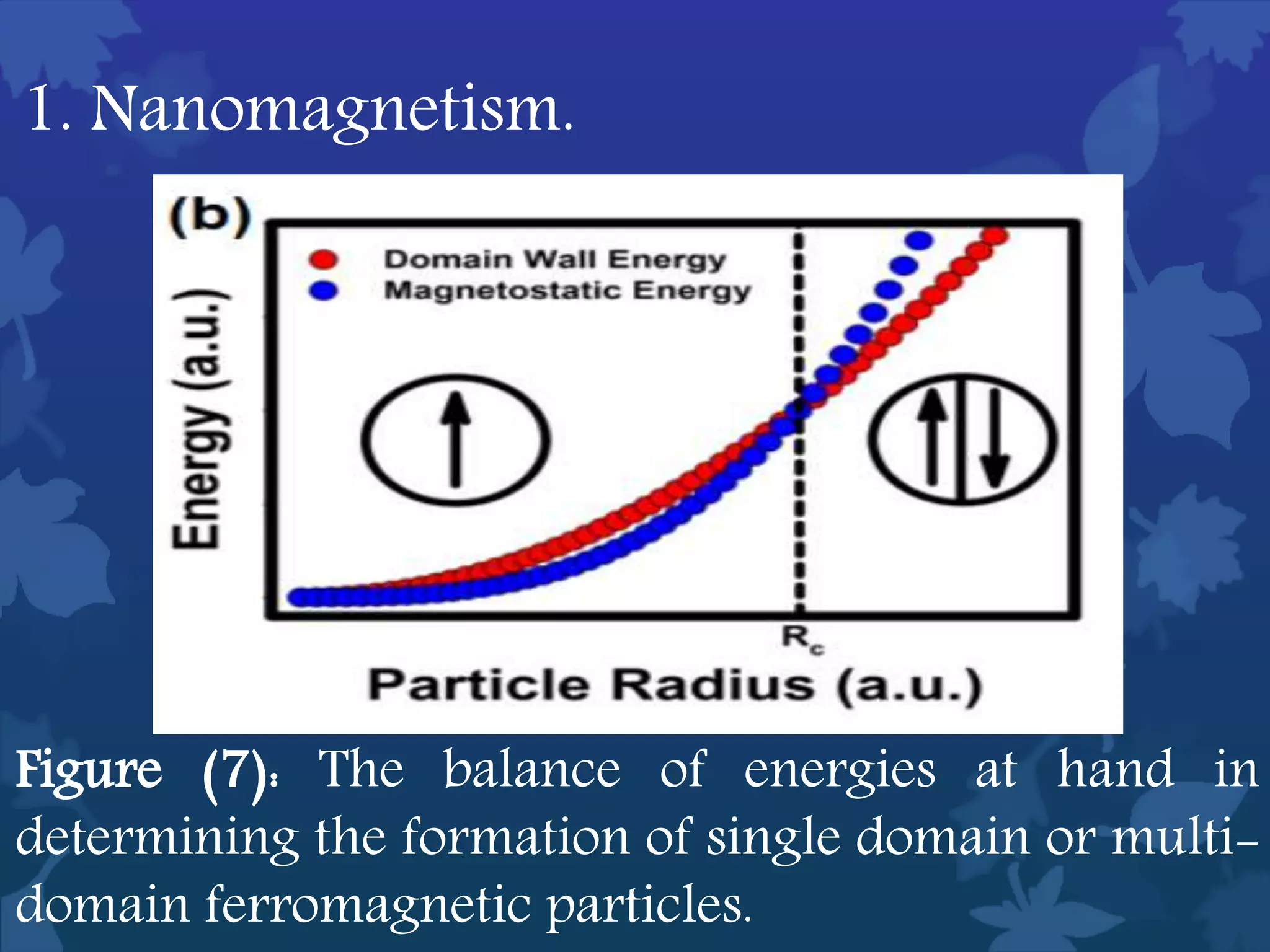
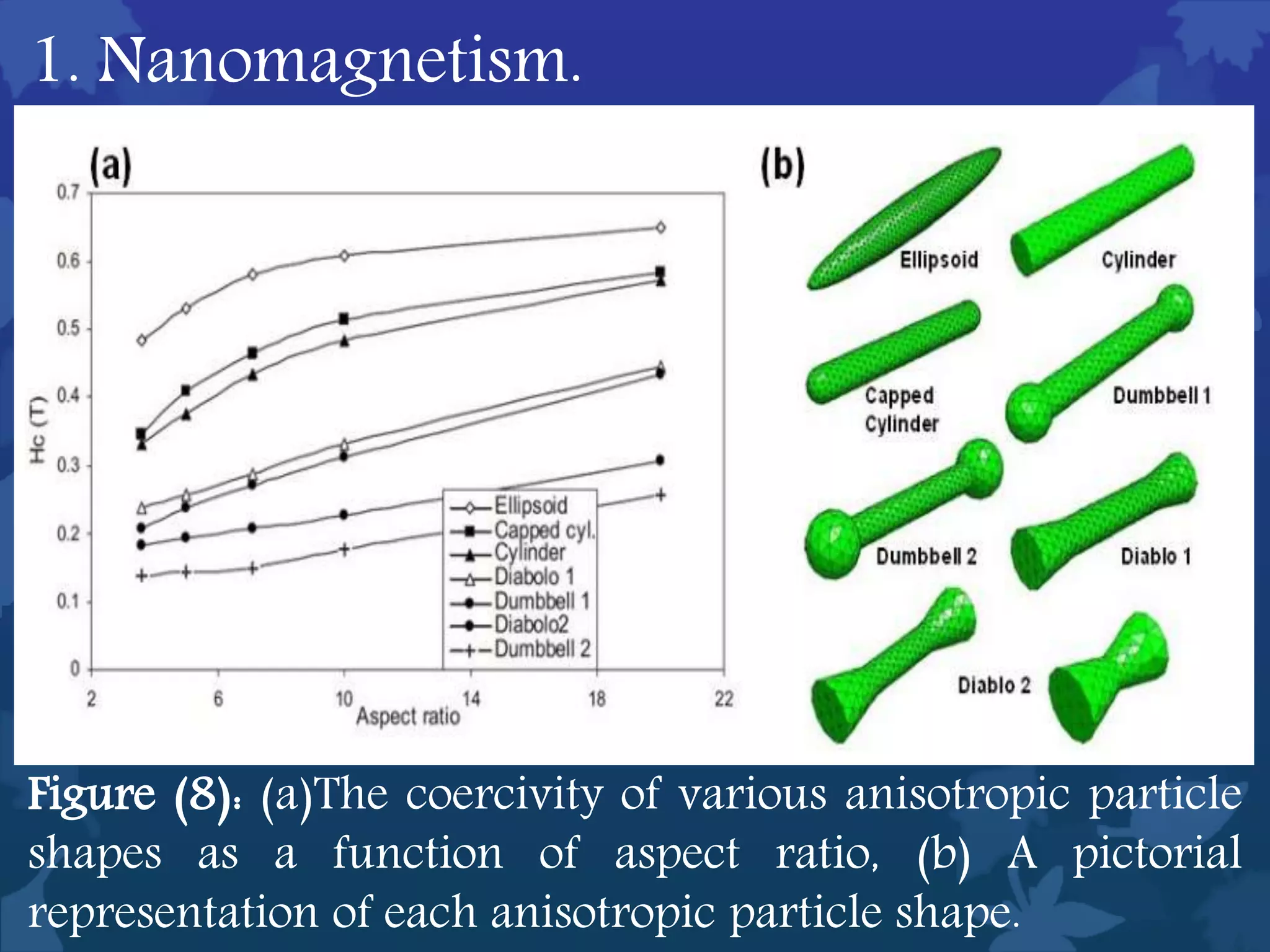
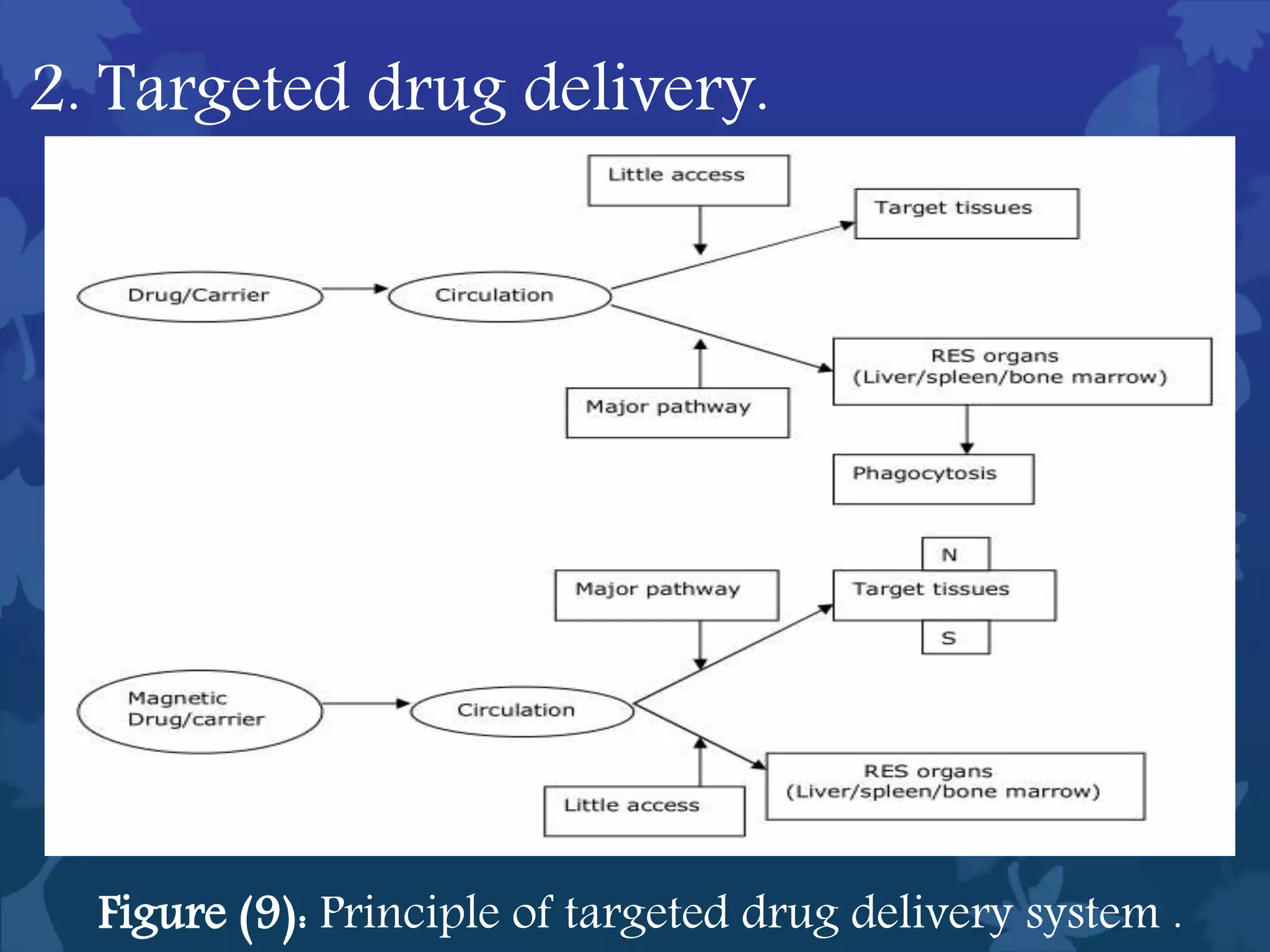
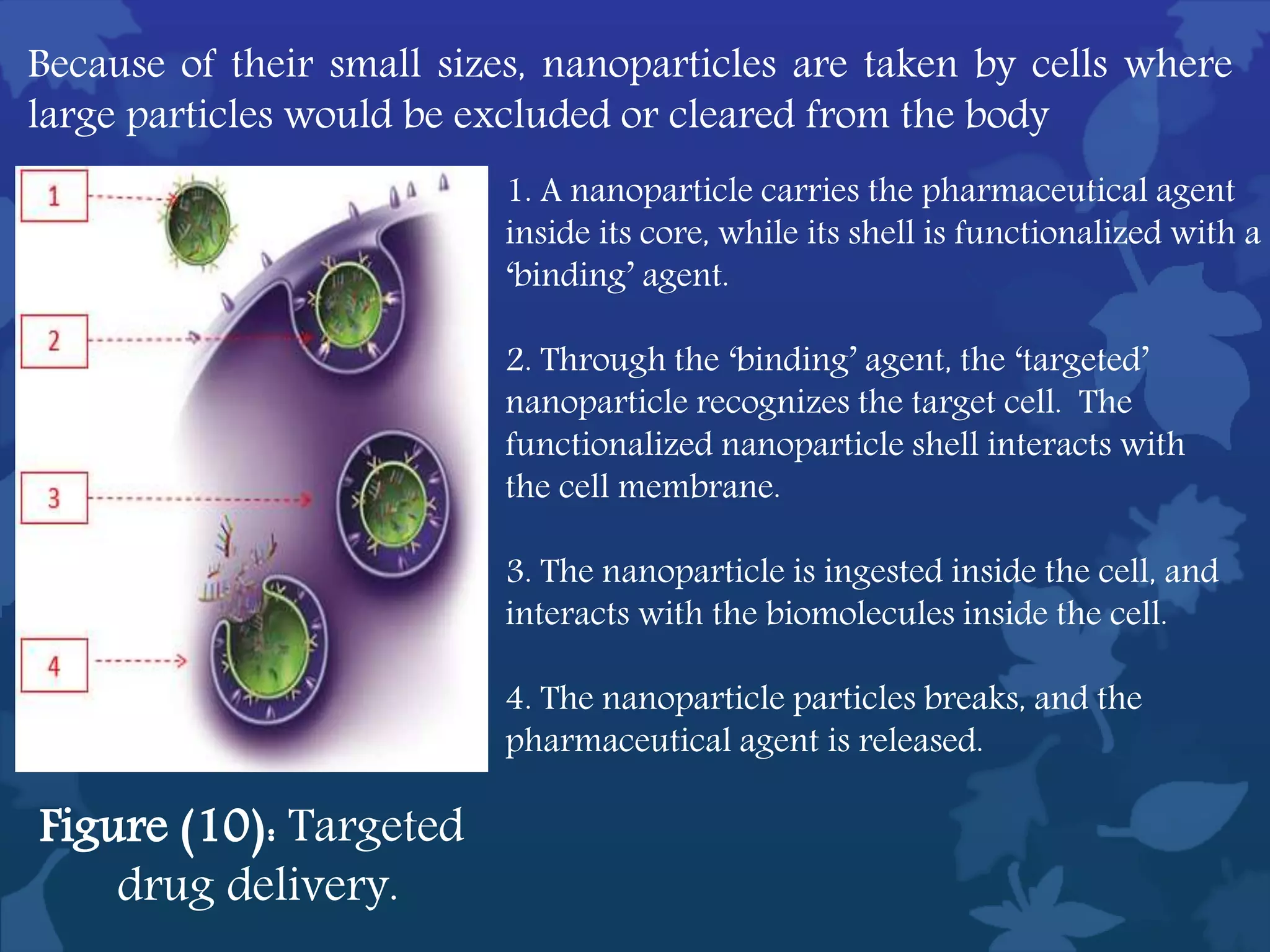
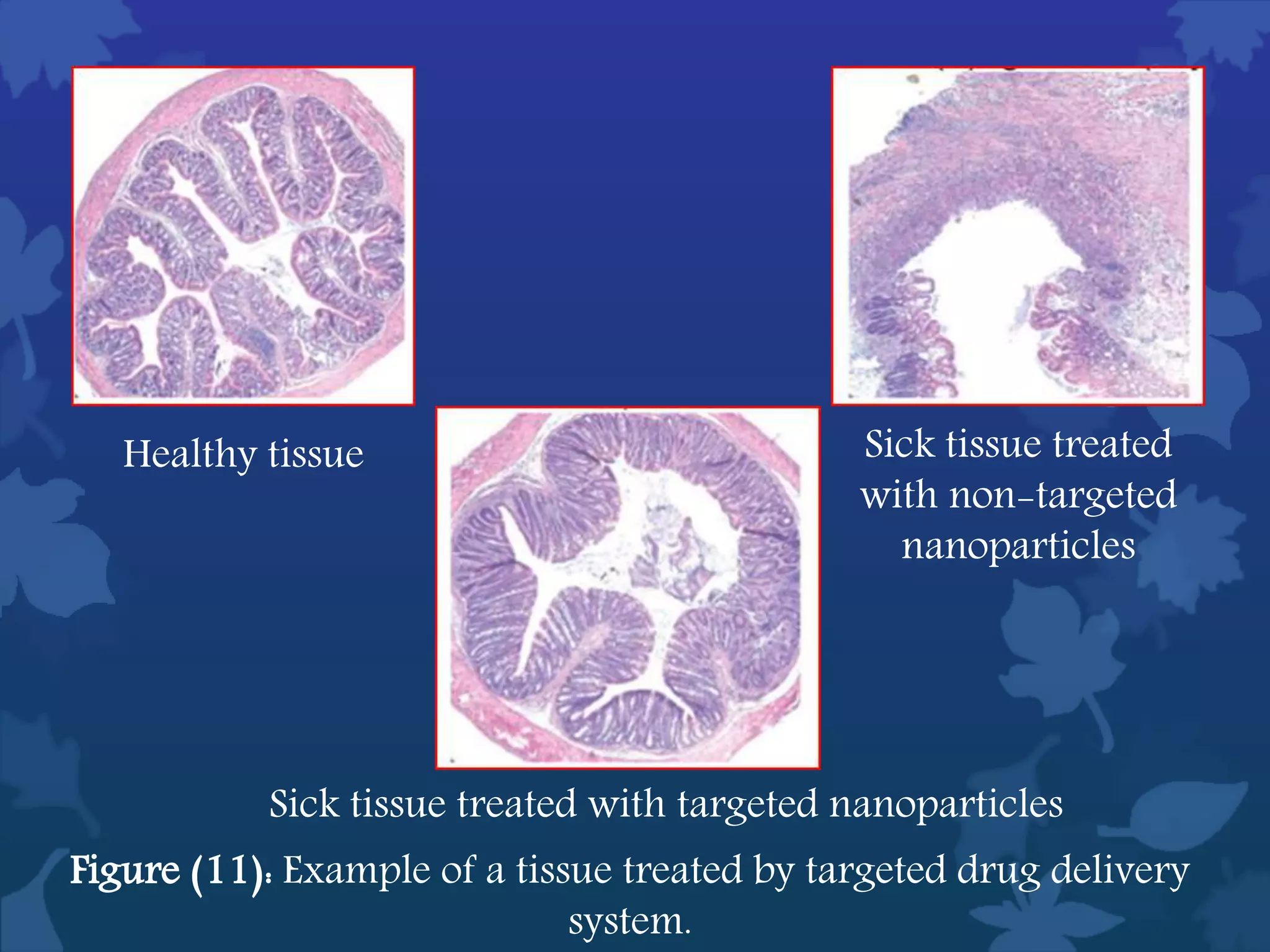
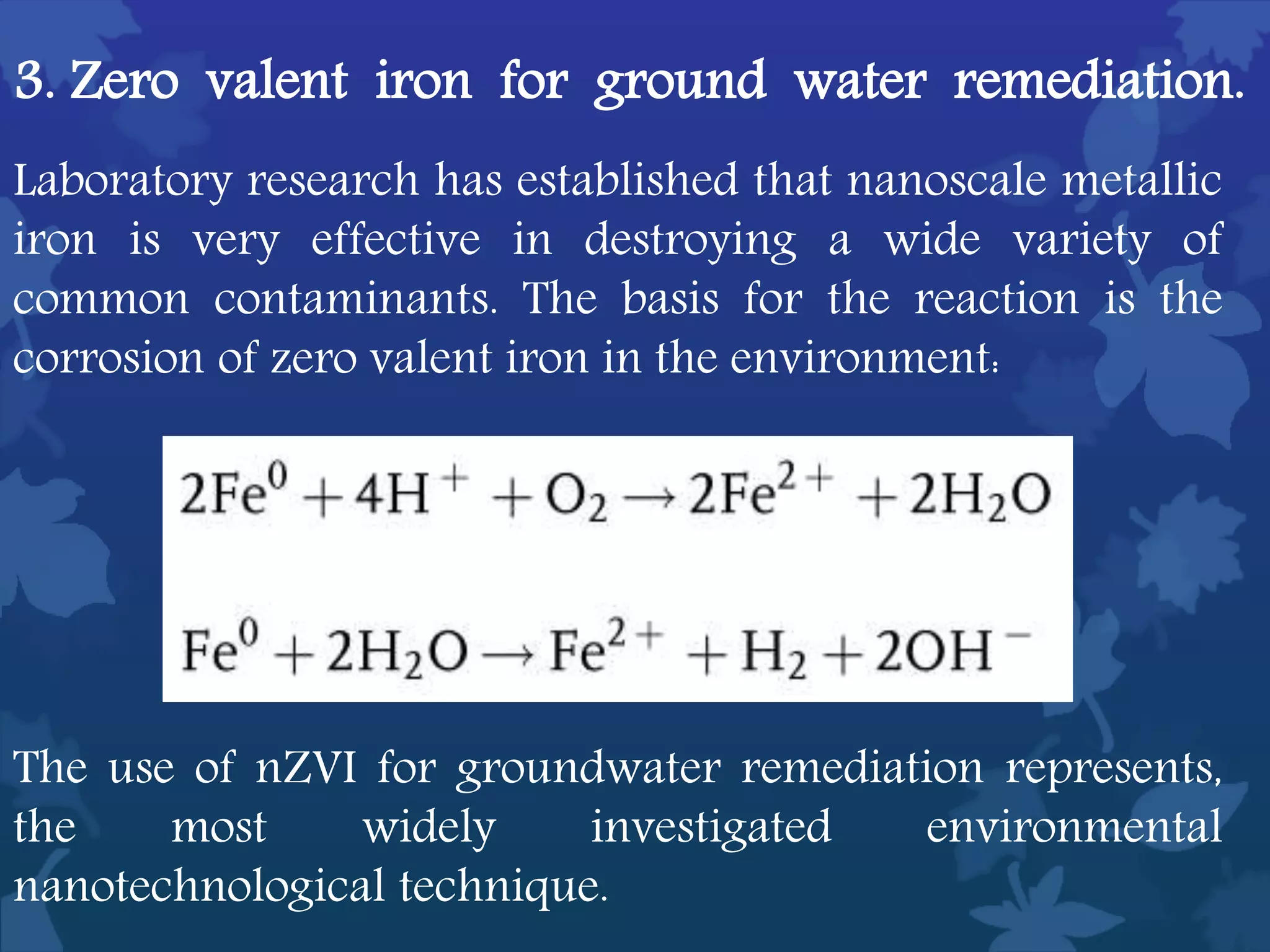
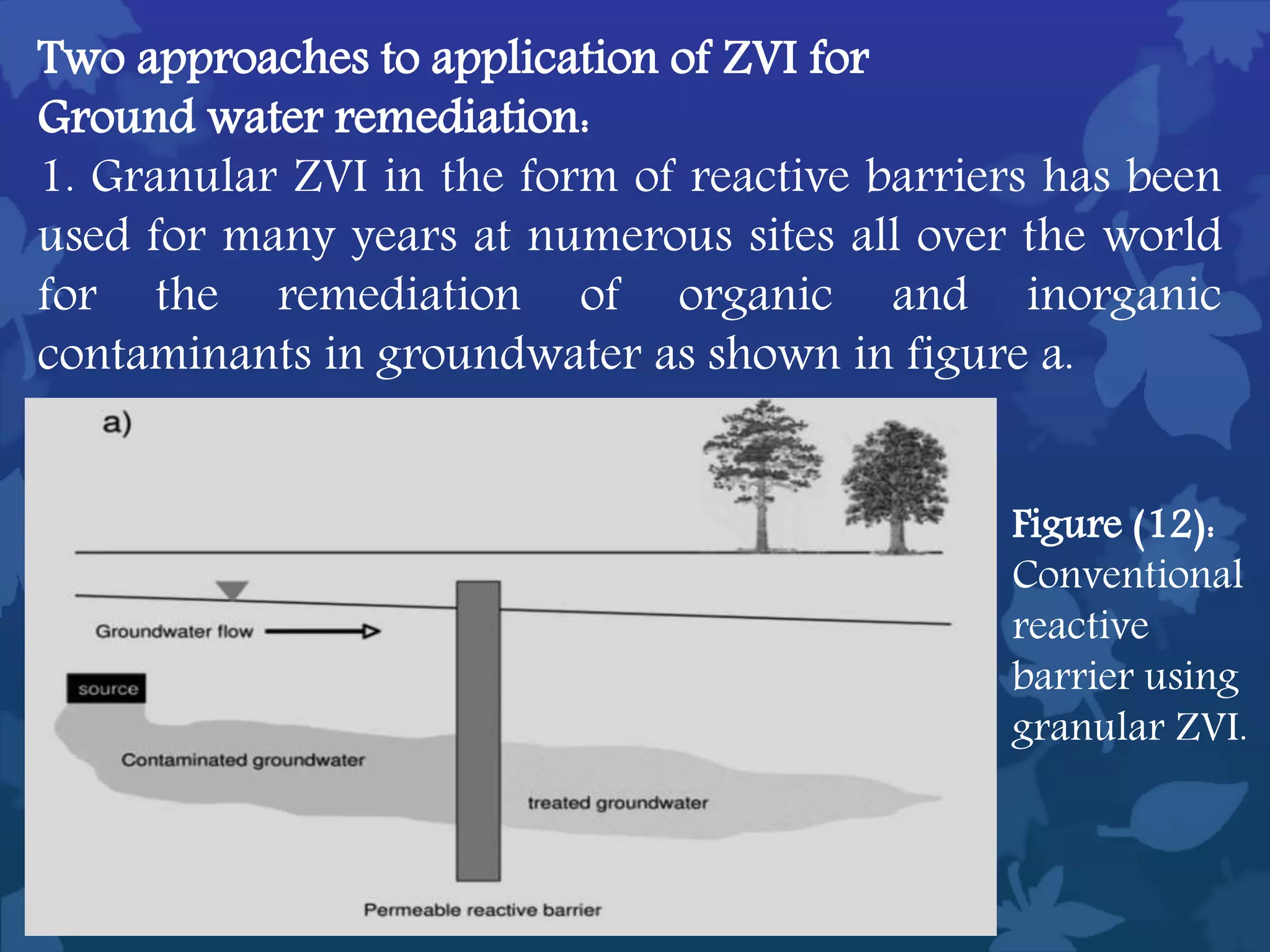
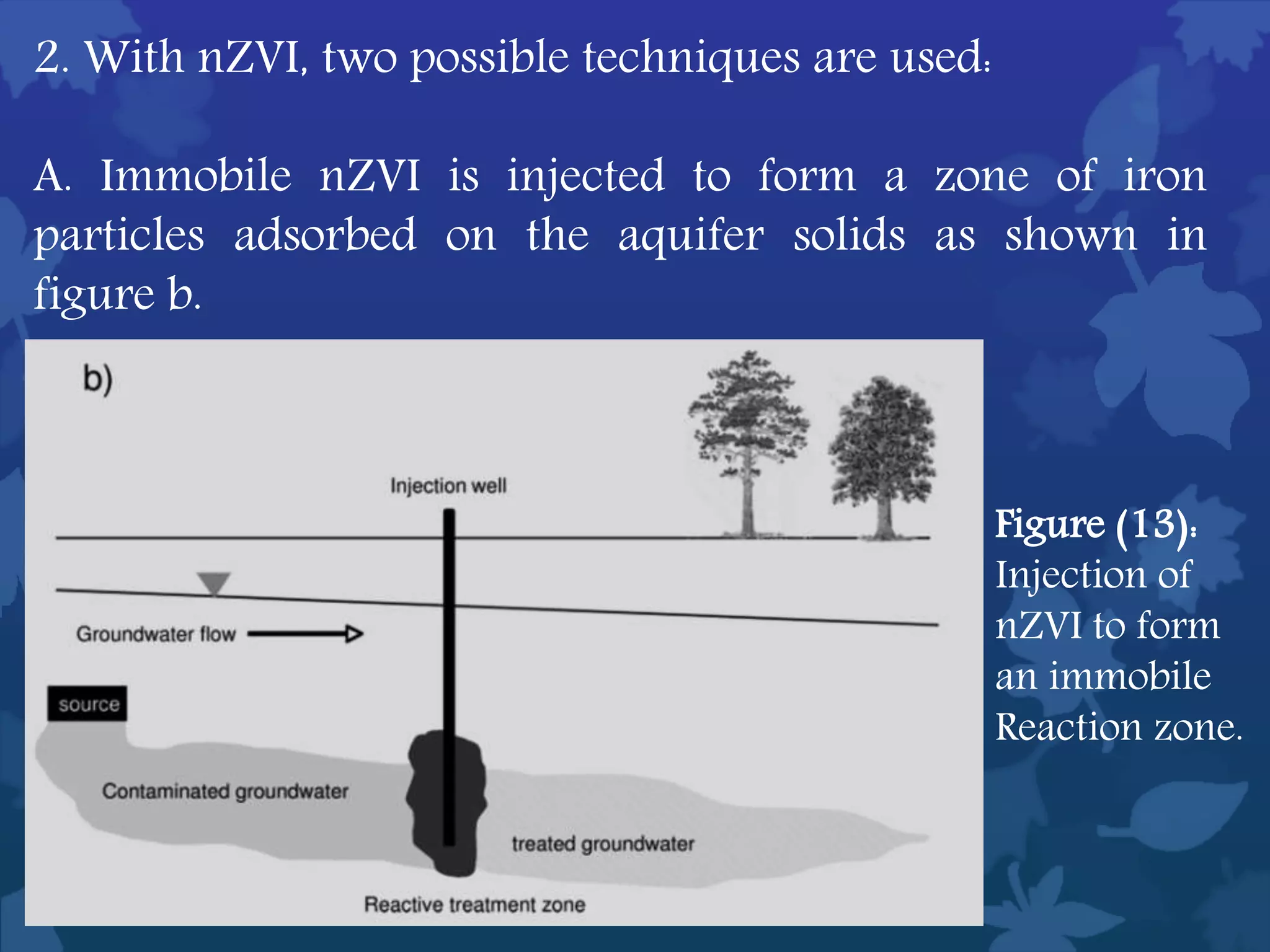
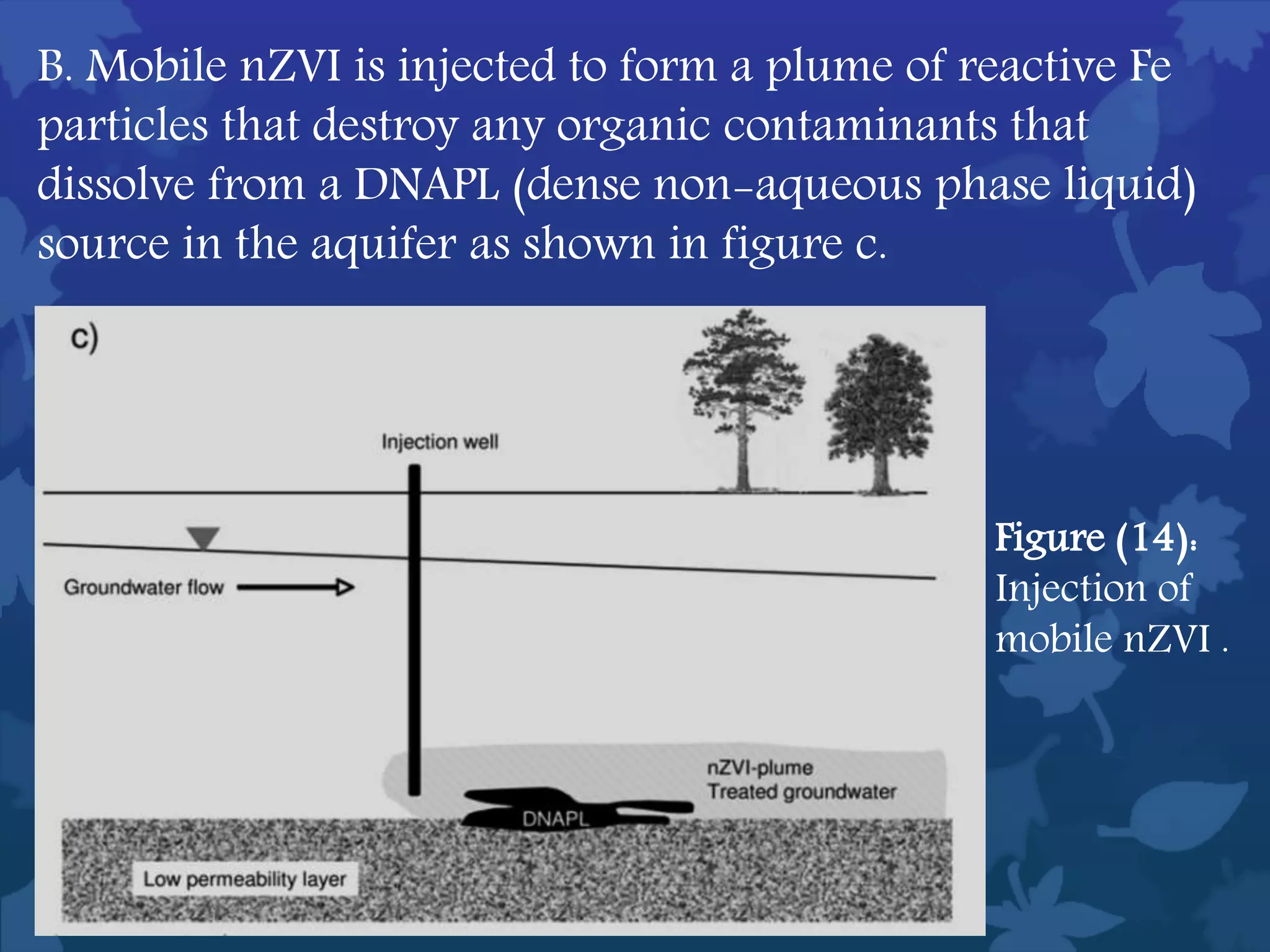
This document discusses ferromagnetic nanomaterials. It introduces magnetic nanoparticles and their size requirements for various applications. It describes the magnetic properties of ferromagnetic materials and how temperature and magnetic fields affect them. Common ferromagnetic elements are iron, nickel and cobalt. Their magnetic domains can be aligned with external fields. Preparation methods like co-precipitation and thermal decomposition are discussed. Applications include nanomagnetism, targeted drug delivery, and using zero valent iron for groundwater remediation.