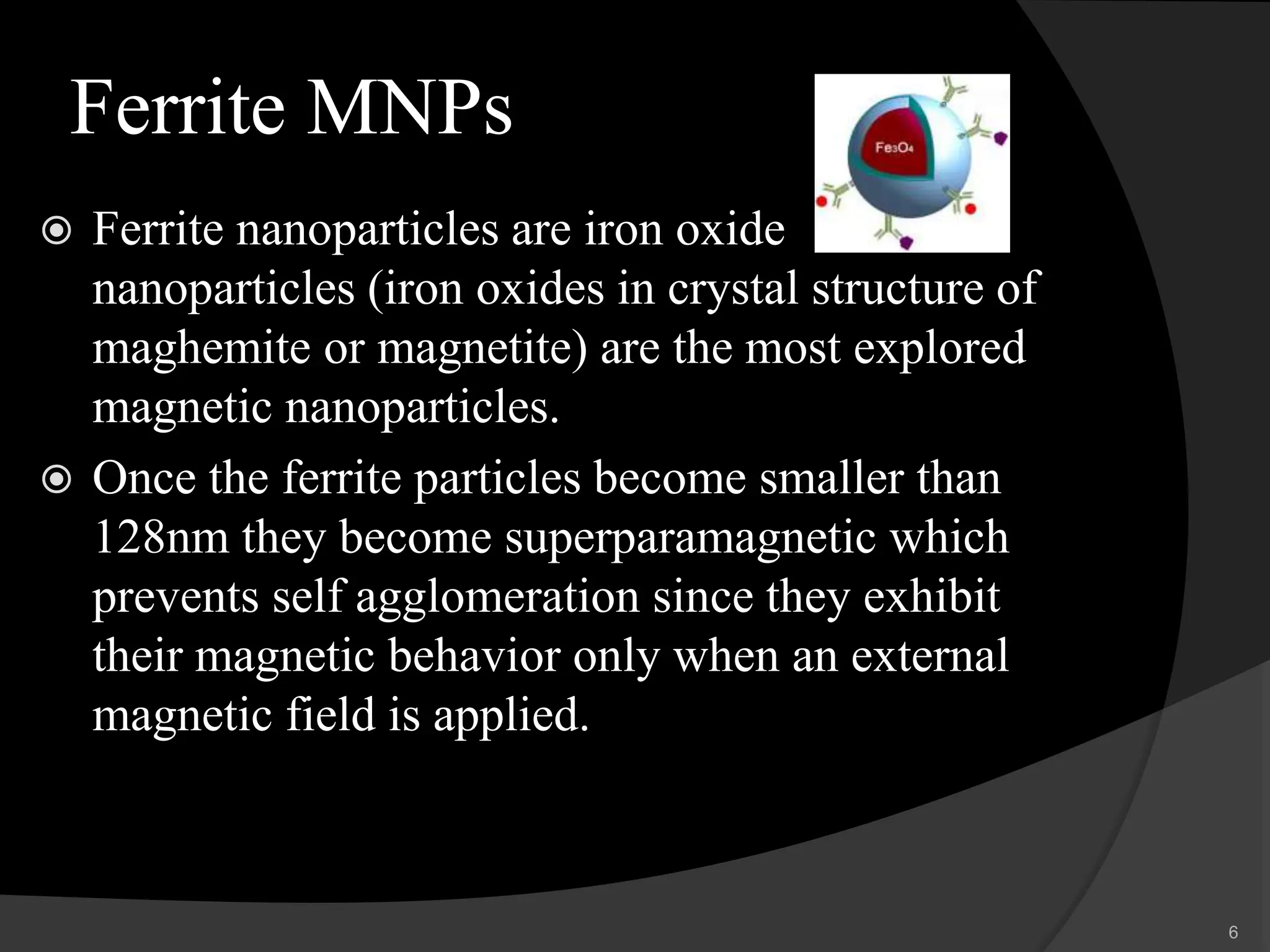
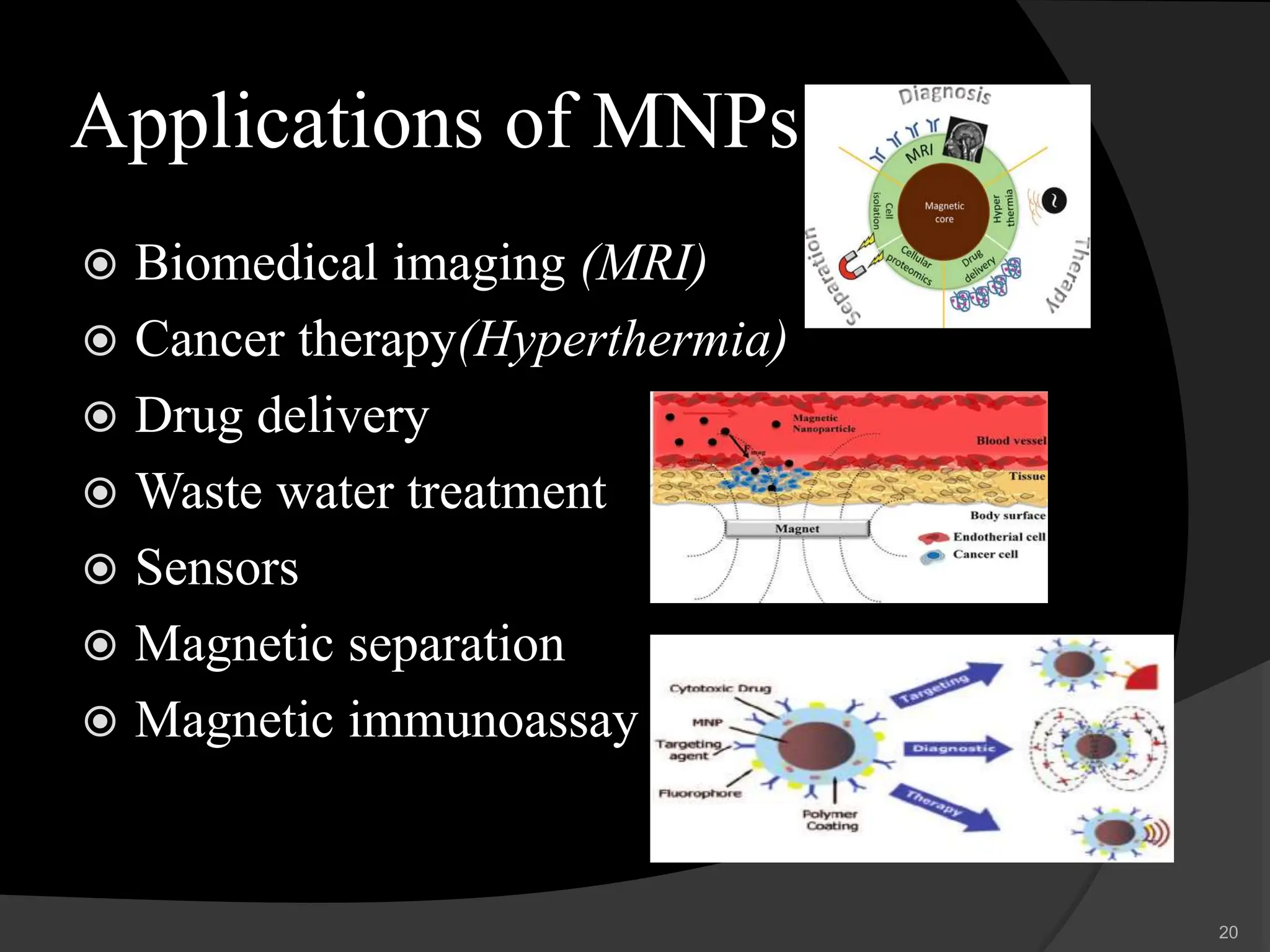
The document discusses magnetic nanoparticles (MNPs), which are nanoparticles that can be manipulated using magnetic fields. It describes various types of MNPs including ferrites, ferrites with a shell, metallic nanoparticles, and metallic nanoparticles with a shell. Common synthesis methods are also summarized, such as co-precipitation, microemulsion, thermal decomposition, and hydrothermal synthesis. Finally, potential applications of MNPs in biomedical imaging, cancer therapy, drug delivery, and other areas are highlighted.