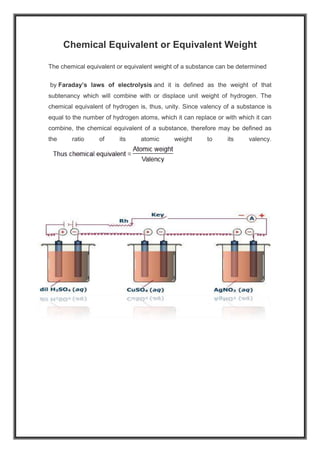
Faraday's laws of electrolysis describe the relationship between electricity passed through an electrolyte and the amount of chemical deposited at the electrodes. Faraday's first law states that the mass of the chemical deposited is directly proportional to the quantity of electricity passed through the electrolyte. Faraday's second law states that when the same quantity of electricity is passed through different electrolytes, the mass of substance deposited is directly proportional to its chemical equivalent and inversely proportional to its valency. The chemical equivalent of a substance is defined as its atomic weight divided by its valency.