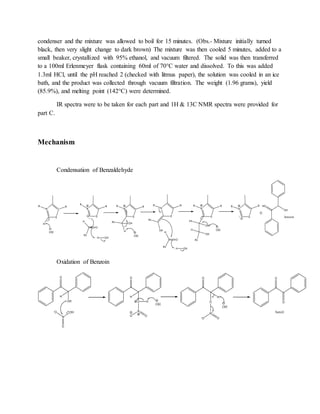
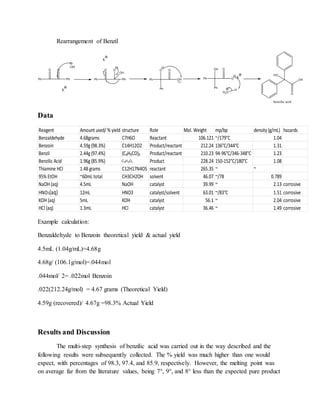
The document summarizes an organic chemistry experiment involving a multi-step synthesis of benzilic acid from benzaldehyde. The synthesis proceeded in three steps: (1) benzaldehyde was condensed to form benzoin, (2) benzoin was oxidized to form benzil, and (3) benzil underwent rearrangement and acidification to form benzilic acid. While product yields were high between 98-86%, melting points were lower than expected, possibly due to experimental errors. NMR spectroscopy confirmed the structure of the final product, benzilic acid.