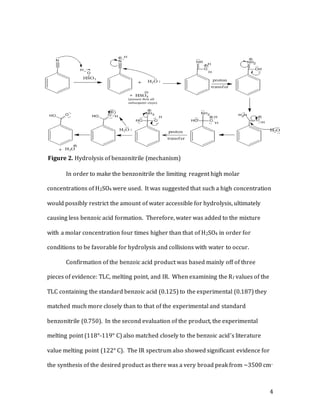
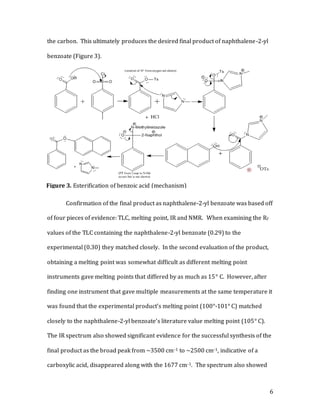
The document describes a two-step synthesis of naphthalen-2-yl benzoate. First, benzonitrile was hydrolyzed using sulfuric acid to produce benzoic acid. This was confirmed using TLC, melting point, and IR spectroscopy. Second, benzoic acid was esterified with 2-naphthol using tosyl chloride and N-methylimidazole as a catalyst to produce the target compound naphthalen-2-yl benzoate. The product was confirmed using TLC, melting point, IR, and NMR spectroscopy. The overall synthesis involved a hydrolysis followed by an esterification to convert the starting nitrile to the final est