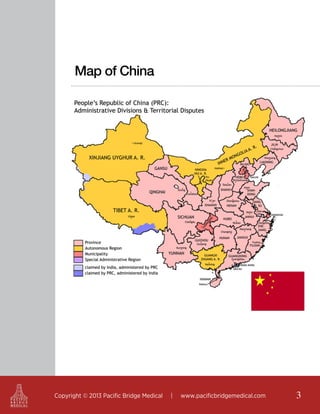
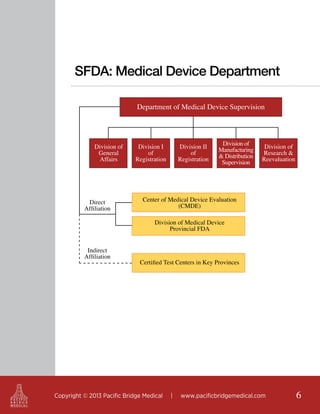
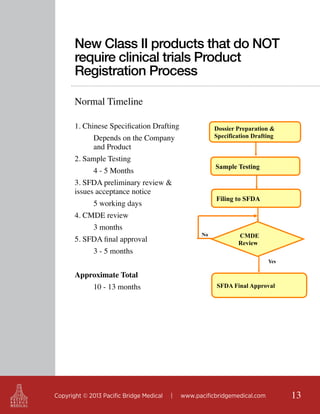
The document outlines the regulatory landscape for medical devices in China, emphasizing that it is the seventh largest market globally and offers details on the roles of key regulatory authorities such as the State Food and Drug Administration (SFDA). It highlights the classifications of medical devices, the registration process, and the requirements for legal and technical documentation required for compliance with Chinese regulations. Additionally, it discusses the importance of product standards, adverse event reporting, and the potential need for clinical trials for products not yet registered in China.