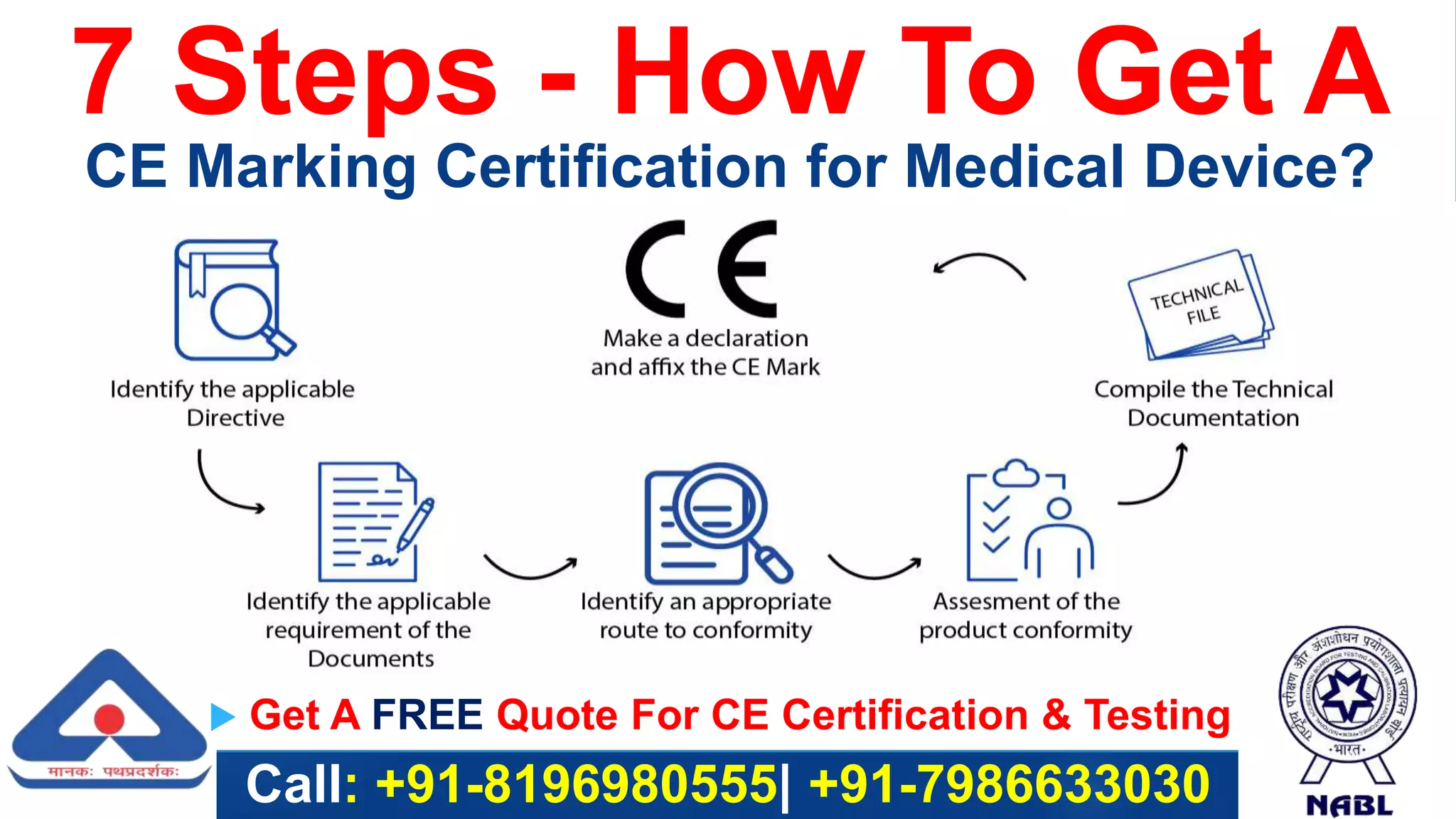
The document outlines the 7 steps to obtain CE marking certification for a medical device: 1) Classify the device, 2) Identify relevant standards and regulations, 3) Compile technical documentation and testing results, 4) Appoint a European authorized representative if located outside the EU, 5) Obtain certification from a notified body for class II/III devices or self-certify for class I, 6) Affix the CE marking, and 7) Comply with any national requirements. The service described can assist manufacturers with all aspects of the certification process.