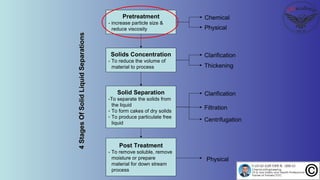
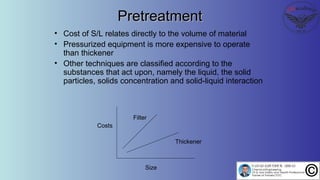
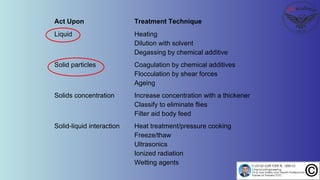
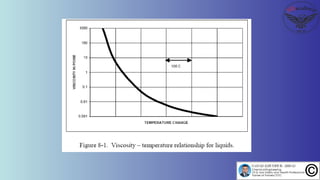
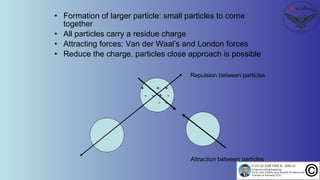
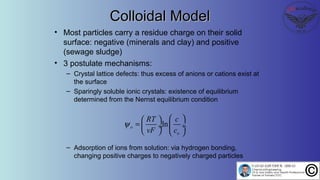
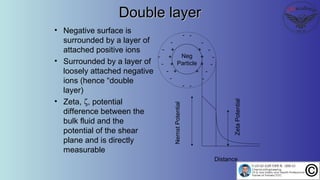
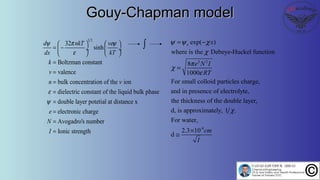
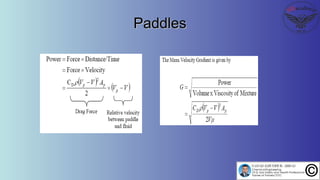
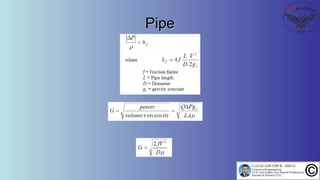
The document discusses the four stages of solid-liquid separations: pretreatment, solid separation, post-treatment, and the costs associated with various techniques. It covers methods for improving particle agglomeration and separation through coagulation and flocculation, as well as treatment techniques affecting viscosity. Additionally, it explains the significance of particle charges and the mechanisms involved in aggregation rates.