Liquid membranes can be either immobilized liquid membranes (ILM), also called supported liquid membranes (SLM), or emulsion liquid membranes (ELM). ILMs are made by immersing a thin porous solid film with a liquid, allowing molecules to diffuse through the liquid in the pores. ELMs contain small liquid globules of one liquid suspended in another liquid medium. Liquid membranes allow separation through passive diffusion, facilitated transport using a carrier molecule, or coupled transport where the carrier couples the flow of two species. Common applications include removal of specific ions, gases, and organic compounds.

![Introduction
• Types of Membranes :
[3]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-2-2048.jpg)
![Liquid Membrane
• A material for the preparation of a membrane need to be solid only. A
liquid may also act as a separation barrier between two phases or
mediums where the transport occurs by the ‘Solution – diffusion
mechanism ’. [3]
[6]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-3-2048.jpg)

![(1). Immobilized liquid membrane (ILM) :
• Also Called as Supported liquid membrane(SLM)
• Made by immersing a thin porous film of suitable solid substance with a
liquid.
• Molecules diffuse through the
liquid in the pores.
• Membrane thickness is
20 -150 µm
[3]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-5-2048.jpg)
![(2). Emulsion liquid membrane (ELM) :
• An ELM is a liquid droplet which contains number of very small
globules[Droplet - size about 0.5-10 µm] of another liquid within it,
suspended in a liquid medium.
[3]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-6-2048.jpg)
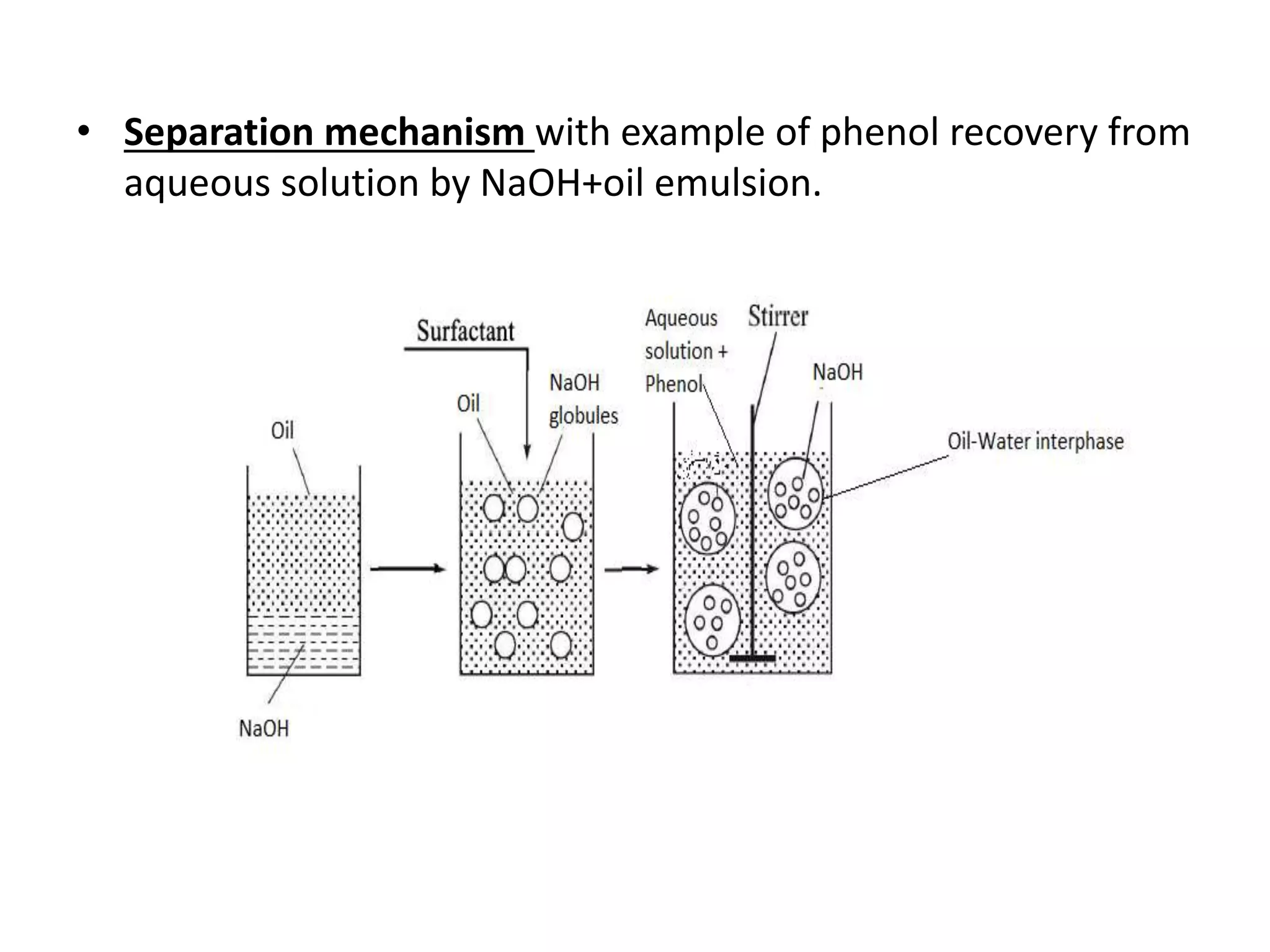
![• The liquid membranes illustrated here are only used in some specific
applications because of the rather low selectivities which is the
differences in solubility and diffusivity.
• Far higher selectivities can be obtained by adding a carrier molecule to the
liquid (membrane) which has a high affinity for one of the solutes in phase
1.
• The carrier accelerates the transport of this specific component. This type
of transport is called 'carrier-mediated‘ transport or facilitated transport.
[1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-8-2048.jpg)
![carrier-mediated transport /
facilitated transport
• When a carrier is present inside the membrane with the ability to complex
with a specific solute the flux of that solute may be enhanced.
• Carrier may be dissolved in the liquid - carrier is mobile.
• Carrier can be bound chemically (covalently) or physically to a solid
polymer - Carrier is fixed and has a very restricted mobility.
[1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-9-2048.jpg)
![• Comparison of diffusivities :
System Mechanism D(cm2/s)
Mobile carrier system The carrier-solute complex
diffuses across the
membrane.
10-5 – 10-7
Gel system The carrier-solute complex
diffuses across the
membrane.
10-6 – 10-8
Fixed carrier The solute jumps from one
site to the other.
> 10-7
[1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-10-2048.jpg)
![Types of transport mechanism in liquid membrane :
(1). Passive diffusion :
• Passive diffusion dueto concentration gradient.
[2]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-11-2048.jpg)
![(2). Facilitated transport :
• The liquid membrane phase contains a carrier agent that chemically
combines with the feed solute to be transported.
[2]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-12-2048.jpg)
![(3). Coupled transport :
• The carrier agent couples the flow of two species. Because of this
coupling, one of the species can be moved against its concentration
gradient, provided the concentration gradient of the second coupled
species is sufficiently large.
[2]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-13-2048.jpg)

![Preparation technique Materials
Stretching Polypropylene
polytetrafluoroethylene
Phase inversion Polypropylene
polyethylene
• Materials used for support membrane :
[1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-15-2048.jpg)
![ii. Choice of Organic Solvent :
The organic liquid must be a solvent for both the carrier and the carrier-
solute complex.
Another important factor is the viscosity of the organic phase.
On increasing the carrier concentration, two effects are counteracting.
↑ CC → ↑ Entering flux
↑ CC → ↑ Viscosity → ↓ diffusion coefficient → ↓ diffusion flux
[1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-16-2048.jpg)
![• Some solvents used in Liquid Membrane :
Solvents Viscosity (T=298 K) g.cm-1.s-1
o-dichlorobenzene 0.013
1-octanol 0.076
dibutylphthalate 0.154
o-nitrophenyl octyl ether 0.128
o-nitro diphenylether 0.161
[1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-17-2048.jpg)
![ It is essential for the solubility of the organic phase in the aqueous phase
to be as low as possible.
Even if the solubility meets this requirement, after a finite period of time
process will become unstable.
Emulsification of organic phase [1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-18-2048.jpg)
![ One approach to solve these problems is by gelantion of the liquid
membrane phase.
This means that the liquid film has the properties of a highly swollen
crosslinked polymer (a 'gel') rather than that of a liquid.
Although the diffusion coefficient will be lower in a gel phase compared to
the liquid but the stability of the layer will have been improved.
Polymers which are useful in this process are :
poly vinyl chloride(PVC),
poly acrylonitrile (PAN) and
poly methylmethacrylate (PMMA).
[1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-19-2048.jpg)
![iii. Choice of carrier :
High selectivities are obtained if the carrier is very specific to one solute.
Here are some class of carrier molecules mentioned :
oximes (tertiary),
amines,
crown ethers,
cobalt complexes.
[1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-20-2048.jpg)
![Applications
• Removal of specific ions
-cations (cadmium, copper, nickel, lead)
-anions (nitrate, chromate)
• Removal of gases
-oxygen/nitrogen separation
-removal of H2S, C02, S02, CO, NH3
• Separation of organic liquids
• Removal of phenol [1]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-21-2048.jpg)
![Advantages
• Uphill Transport Characteristic
• High Interfacial Area per Unit Volume
• Low Solvent Loss
• Low possibilities of concentration polarization [5]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-22-2048.jpg)
![Disadvantages
SLM
• Instability of liquid film.
• Resistance due to membrane itself.
ELM
• Anything effecting emulsion stability must be controlled, i.e. ionic
strengths, pH, etc.
• If, for any reason, the membrane does not remain intact during operation,
the separation achieved to that point is destroyed.
• In order to recover the receiving phase we have to broke down the
emulsion. Since in order to make stable emulsion we have to work against
the ease of breaking it back down.
[5]
[4]](https://image.slidesharecdn.com/liquidmembrane-170501171547/75/Liquid-membrane-23-2048.jpg)

