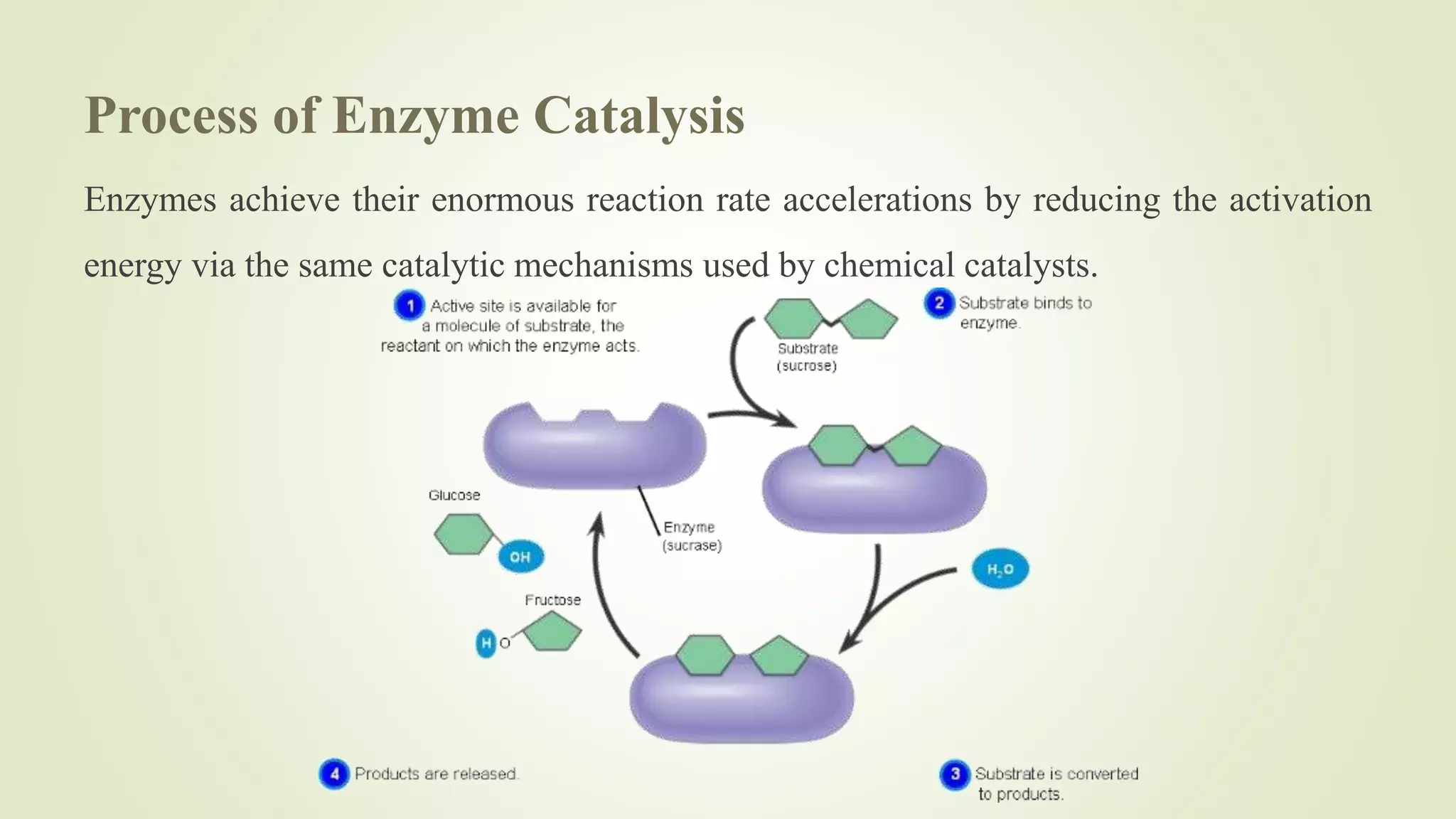
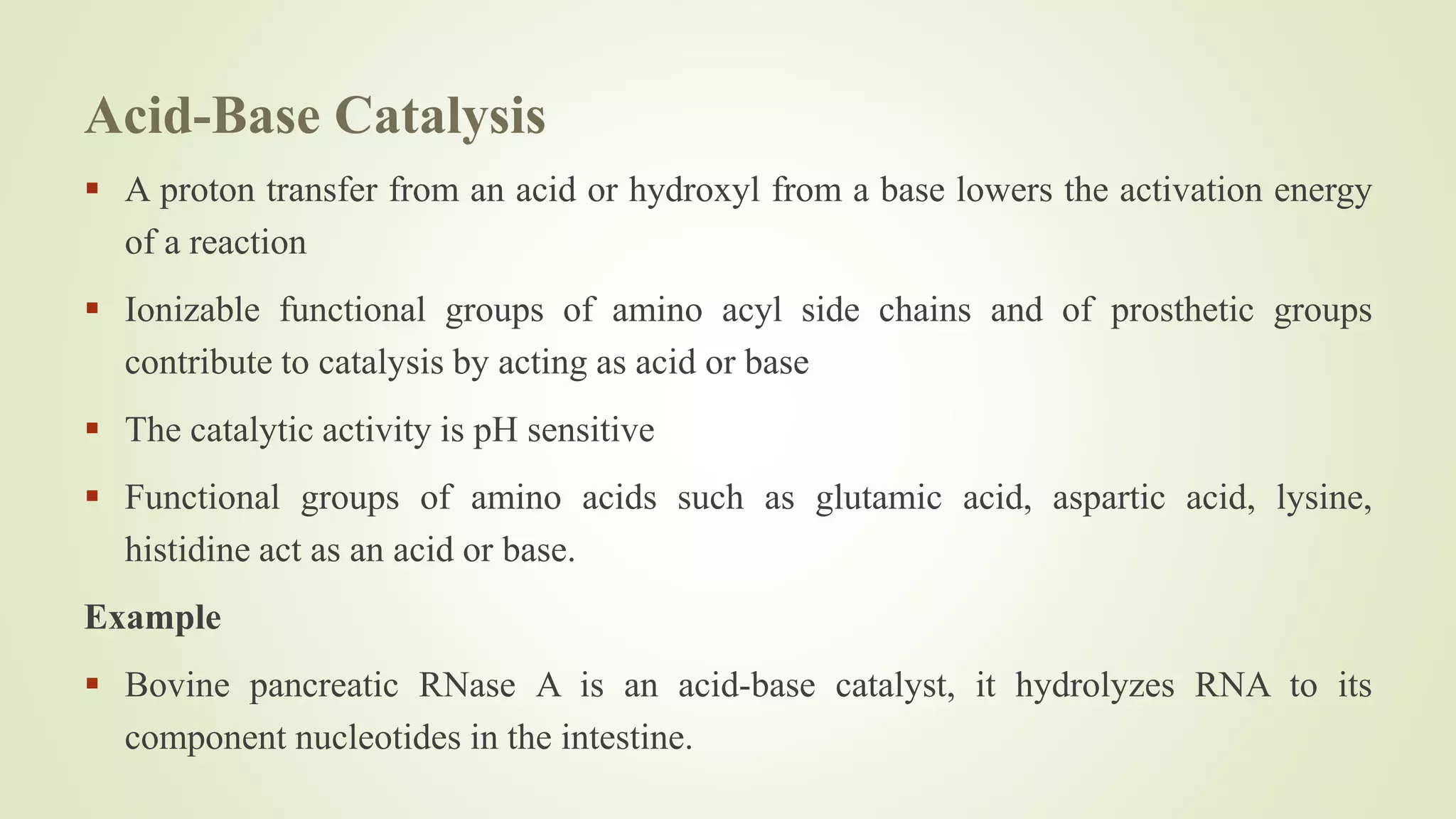
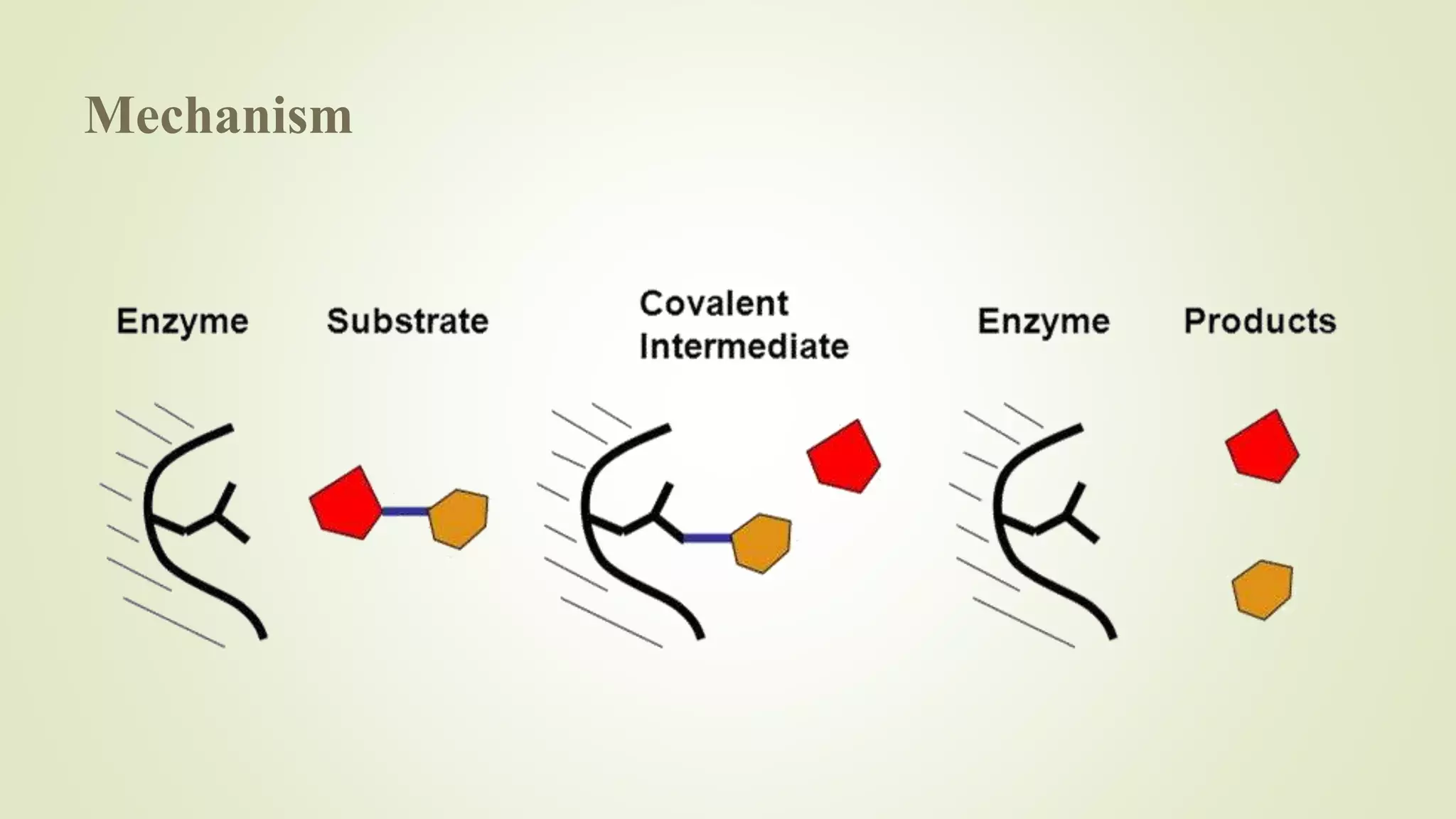
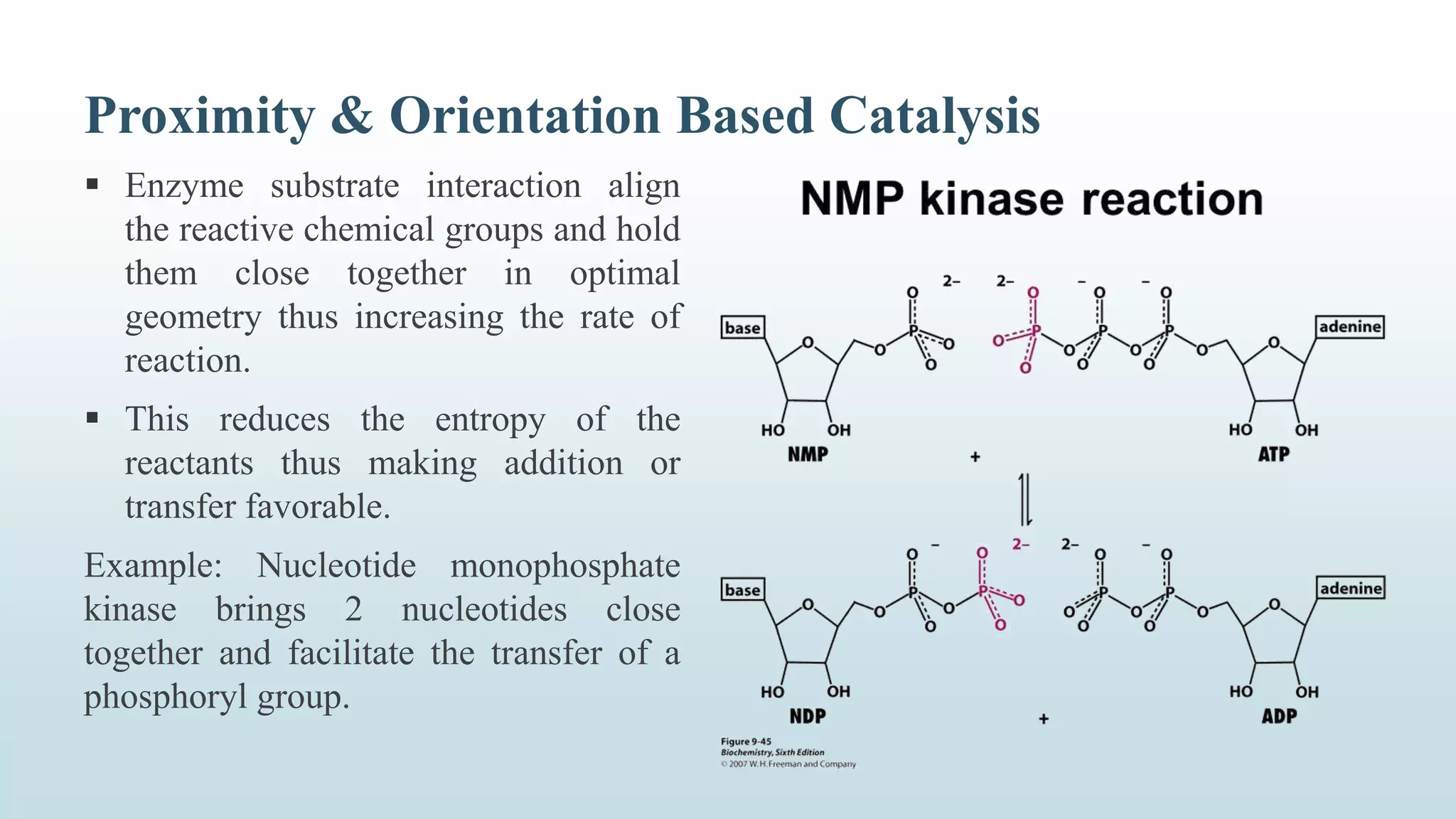
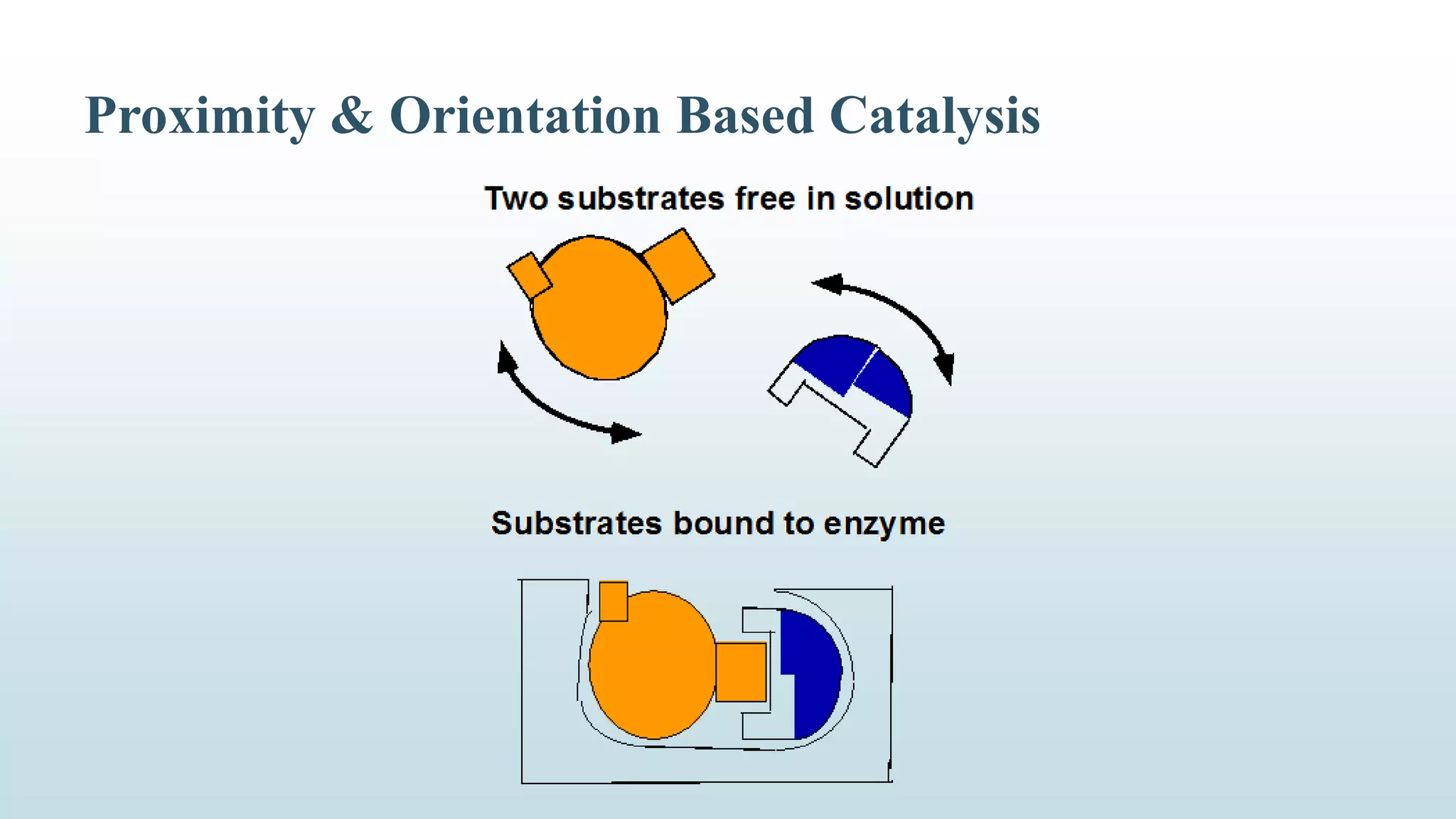
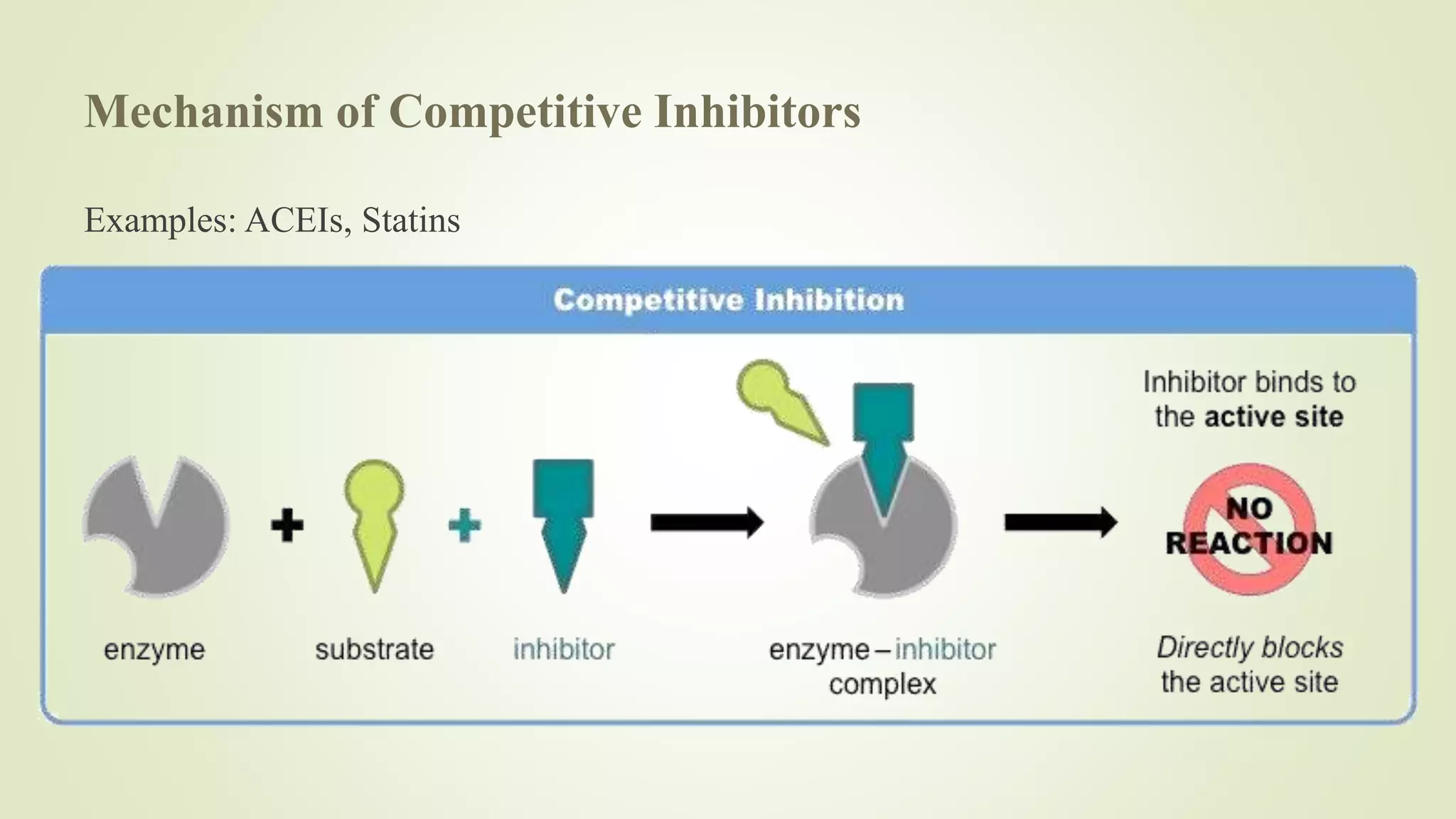
Enzymes catalyze chemical reactions by reducing the activation energy needed for the reaction to occur. They do this using several mechanisms including acid-base catalysis, covalent bond formation, and metal ion catalysis. Enzymes are also able to increase reaction rates by properly orienting substrates. Enzyme activity can be inhibited through various reversible and irreversible mechanisms such as competitive inhibition where an inhibitor binds to the active site, and suicide inhibition where the inhibitor is converted by the enzyme into a tightly-binding form. The Michaelis-Menten model and Lineweaver-Burk plots are commonly used to study enzyme kinetics and inhibition types.


















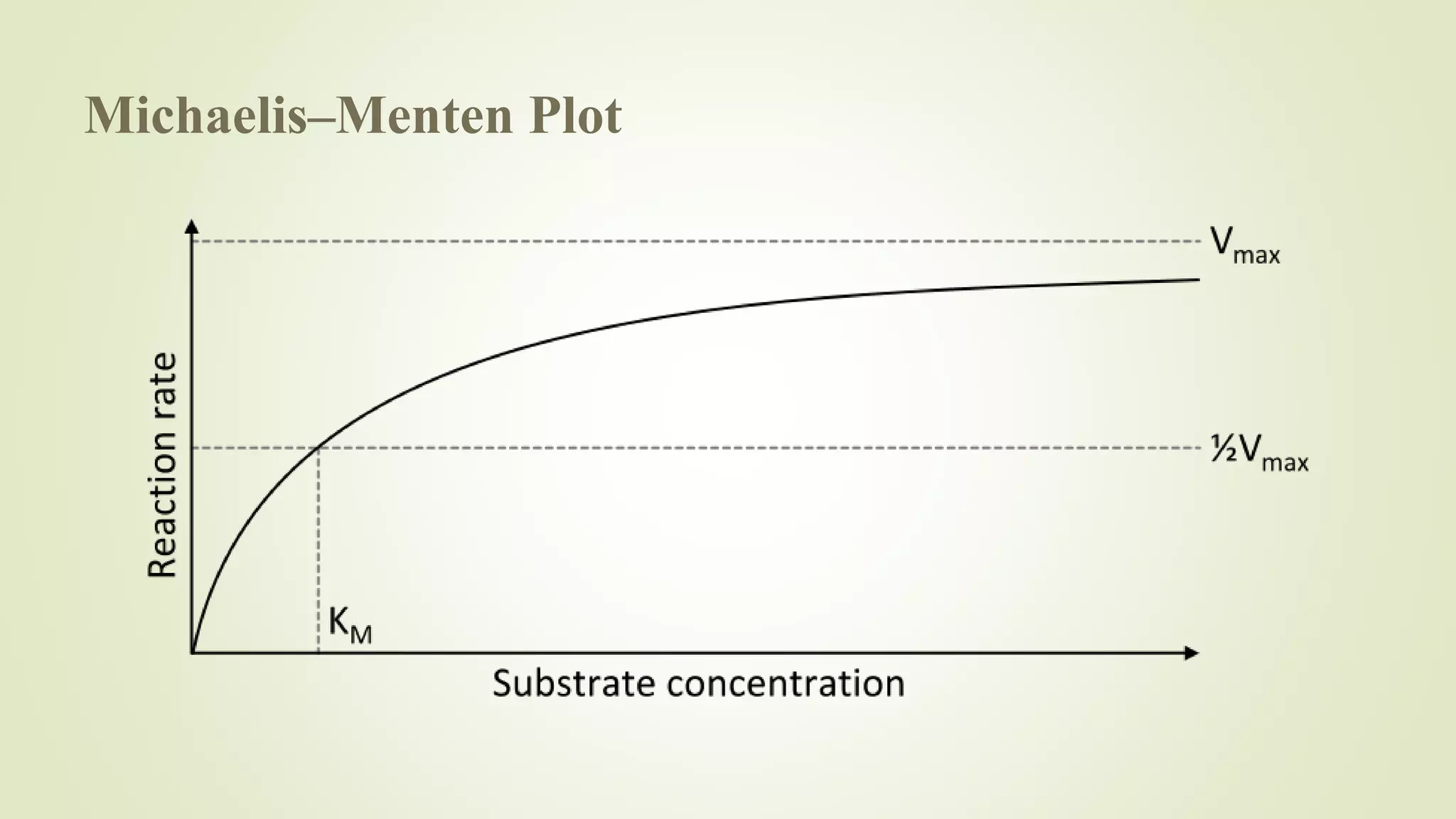
![Michaelis–Menten kinetics
It is named after German biochemist Leonor Michaelis and Canadian physician
Maud Menten.
The model takes the form of an equation describing the rate of enzymatic reactions,
by relating reaction rate to the concentration of a substrate.
The Michaelis-Menten equation has been used to predict the rate of product
formation in enzymatic reactions for more than a century.
Vo= Rate of reaction
Vmax= Maximum reaction rate
[S]= Substrate concentration
KM= Michaelis constant](https://image.slidesharecdn.com/1-200102100325/75/Basics-of-Enzyme-Catalysis-28-2048.jpg)