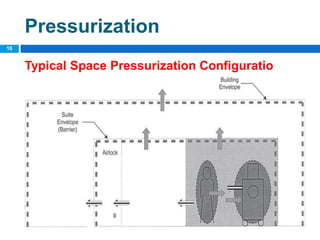
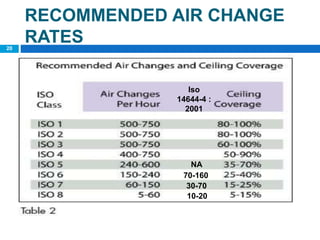
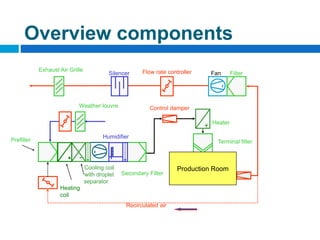
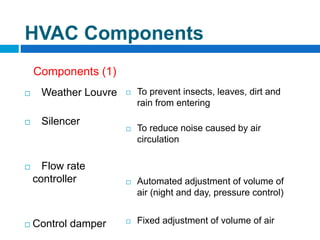
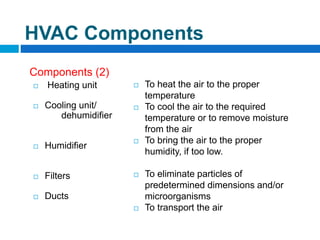
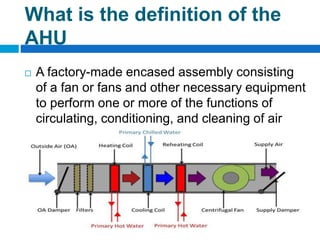
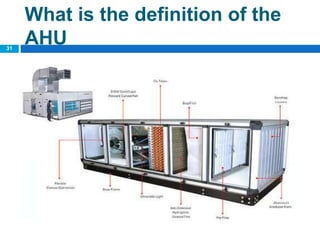
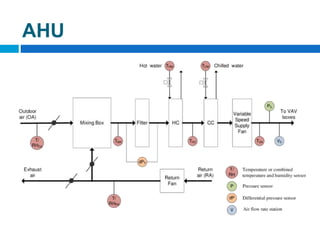
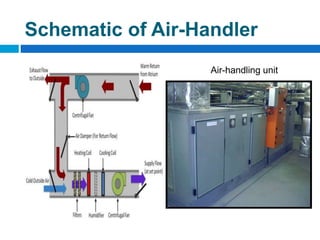
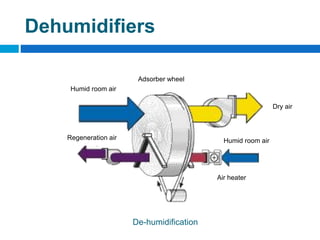
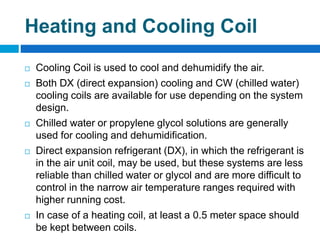
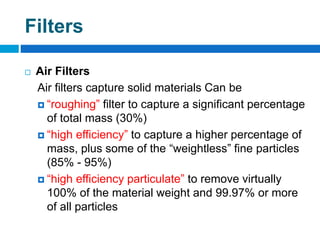
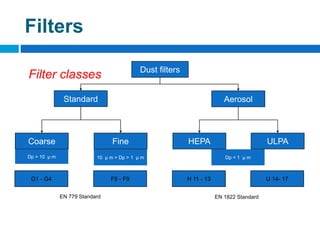
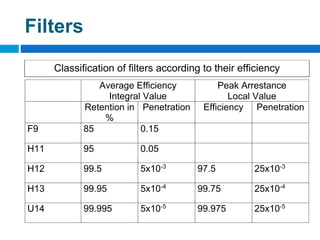
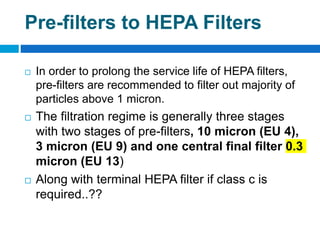
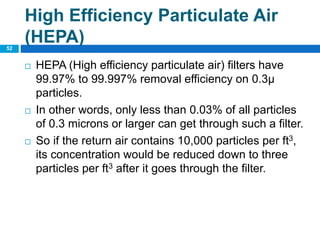
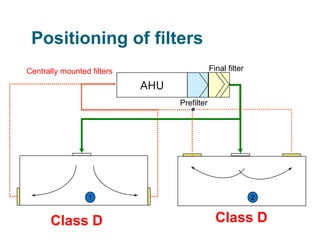
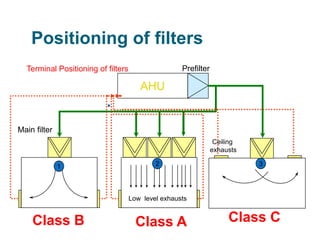
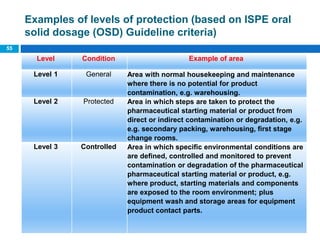
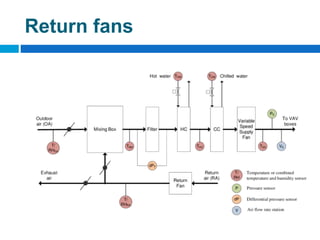
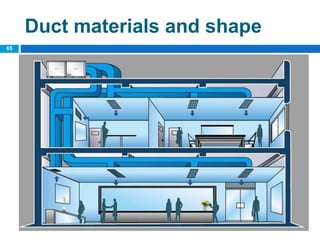
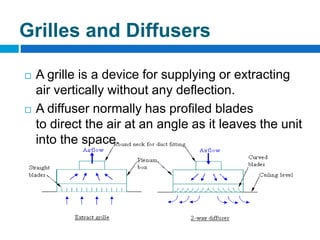
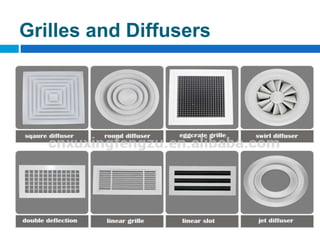
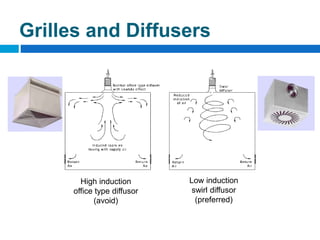
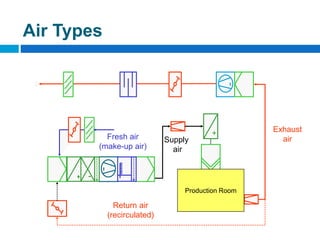
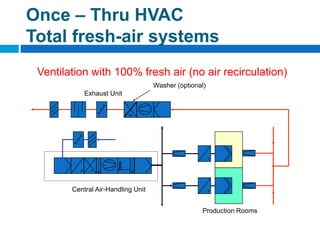
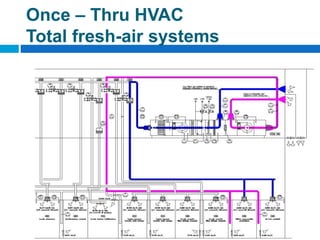
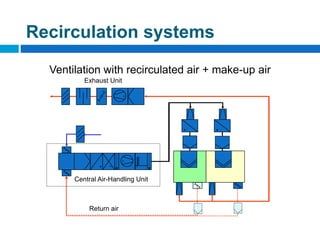
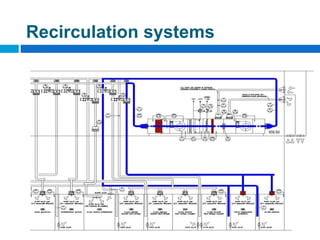
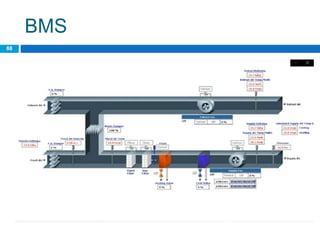
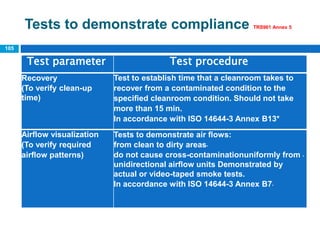
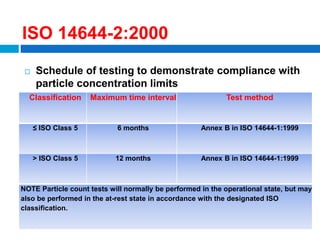
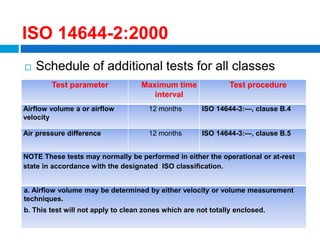
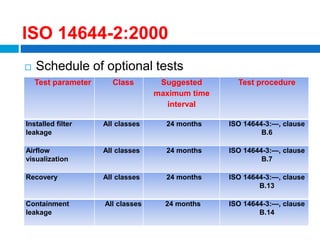
The document outlines the HVAC requirements and design considerations for pharmaceutical facilities, emphasizing the importance of adequate ventilation, air filtration, and temperature and humidity control to maintain product quality and safety. It details the specifications for air handling systems, including filtration types and maintenance protocols, as well as the significance of controlling airborne particles and contaminants. Additionally, it highlights the need for proper building design to ensure cleanliness and effective airflow patterns in compliance with regulatory standards.