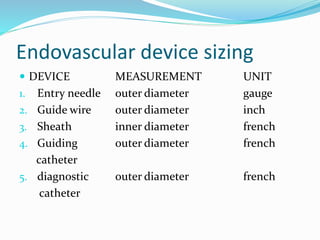
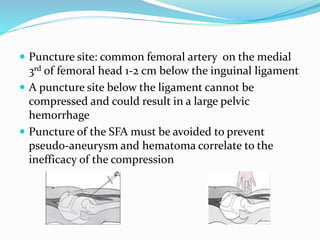
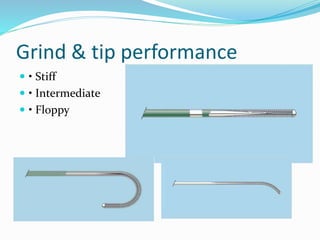
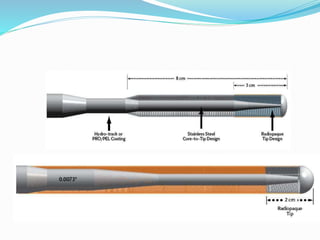
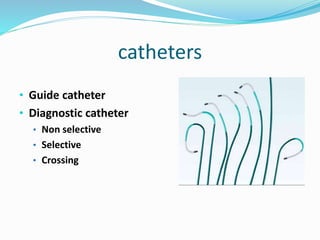
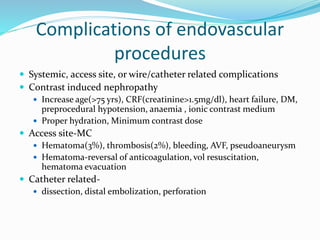
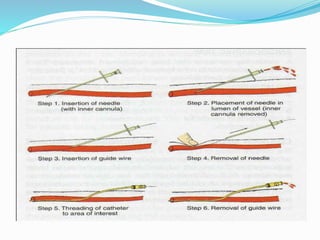
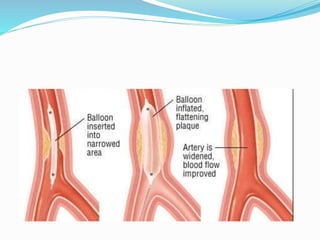
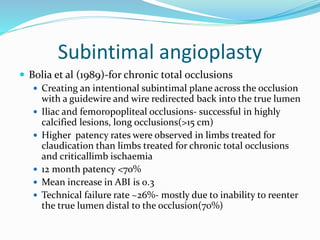
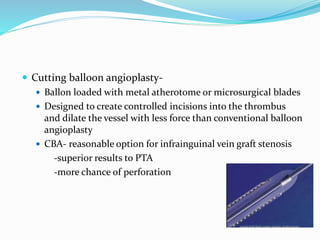
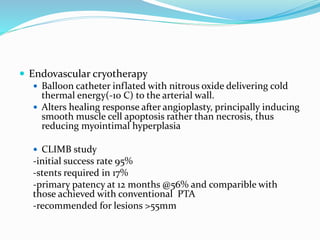
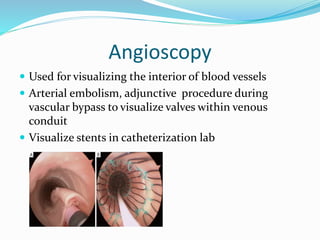
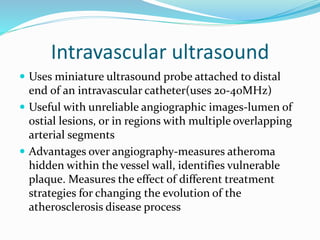
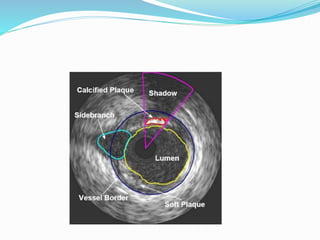
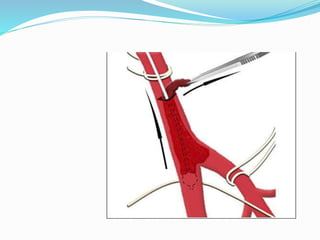
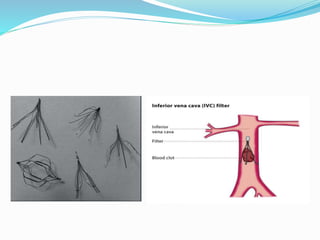
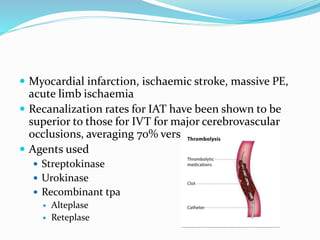
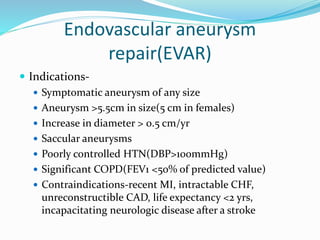
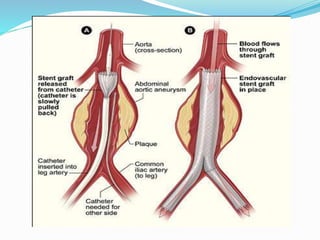
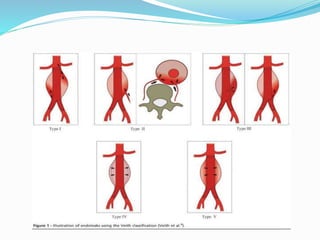
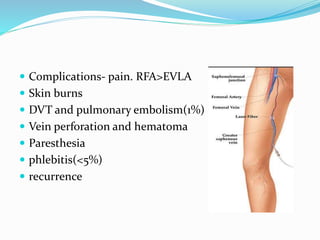
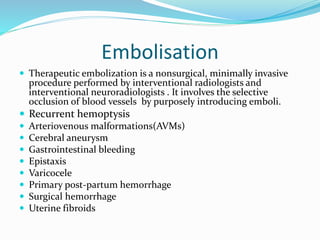
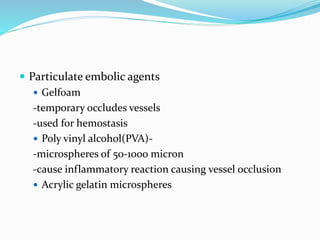
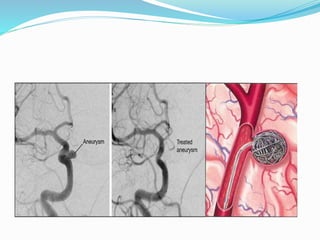
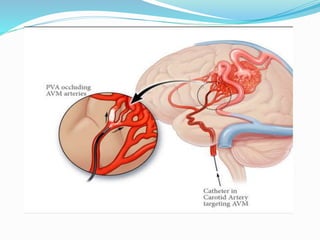
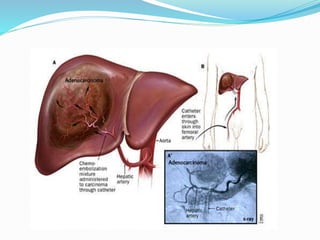
Dr. Anil Meetei presented on endovascular surgery and its various procedures and techniques. Endovascular surgery involves minimally invasive procedures using catheters and instruments inserted into blood vessels. Some key procedures discussed included balloon angioplasty, stenting, atherectomy to remove plaque, thrombolysis to treat clots, and filters to prevent pulmonary embolism. Factors such as device sizing, access points, imaging, and complications were also reviewed.