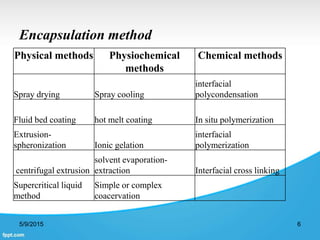
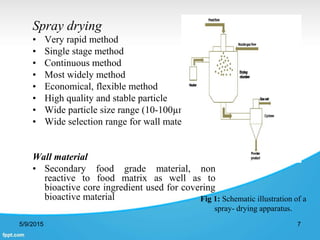
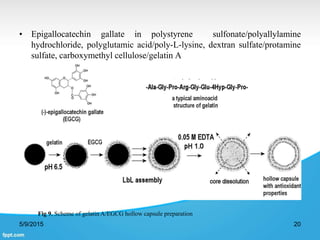
This document discusses encapsulation of natural polyphenolic compounds. It describes various encapsulation methods including spray drying, supercritical fluid techniques, physicochemical methods, and chemical methods. Spray drying is highlighted as the most widely used method for encapsulating polyphenols due to its low cost, flexibility, and ability to produce stable particles. Specific polyphenols that have been encapsulated using different techniques like spray drying, supercritical fluid processes, and complex coacervation are also outlined.