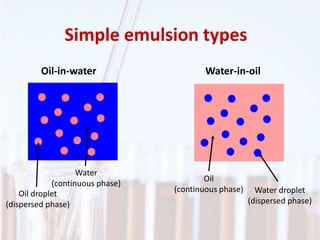
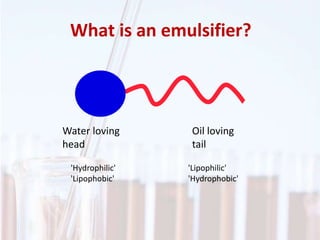
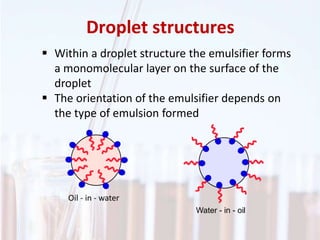
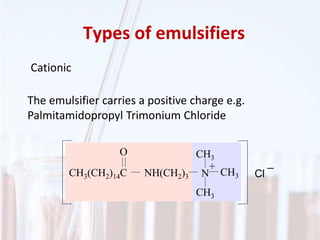
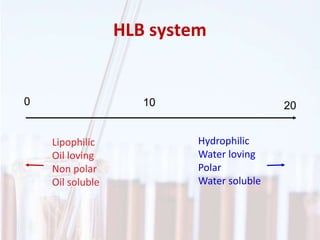
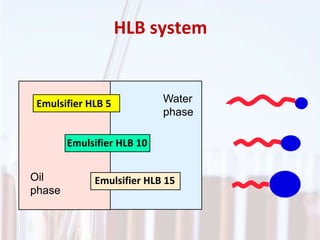
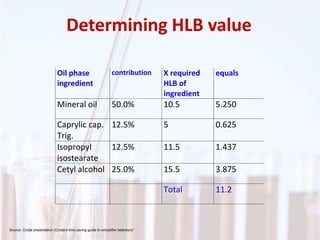
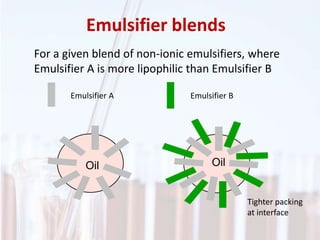
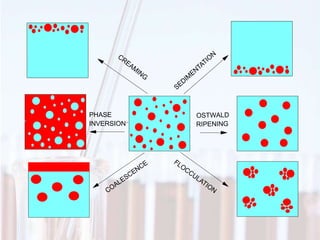
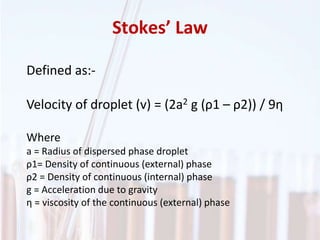
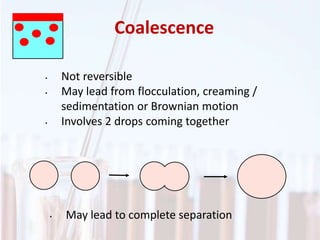
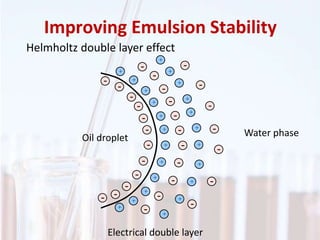
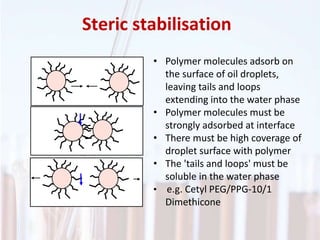
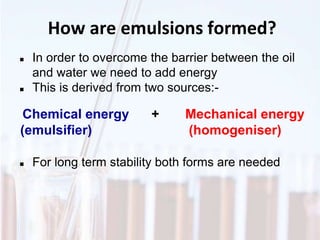
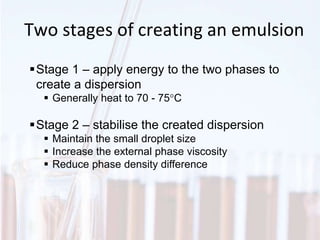
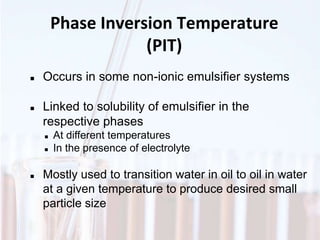
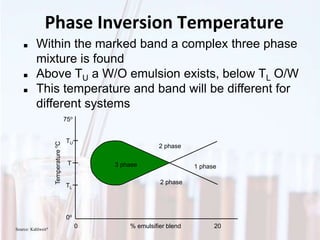
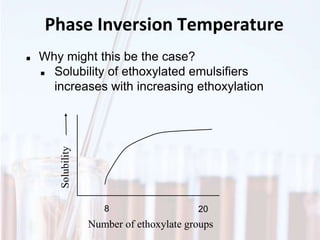
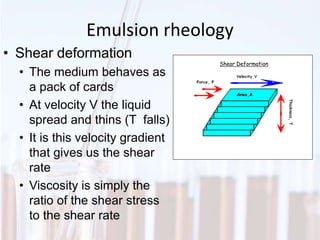
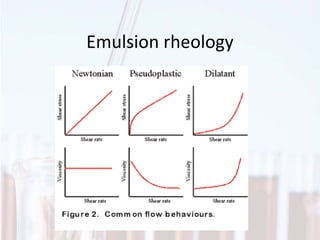
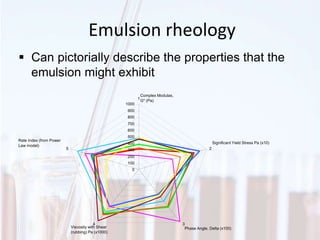
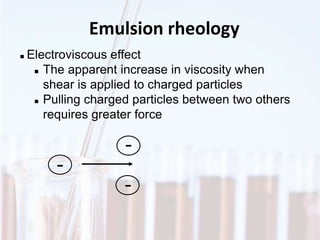
An emulsion is a dispersion of one immiscible liquid within another. Emulsions are thermodynamically unstable but can exist in a metastable state. The stability of an emulsion depends on factors like interfacial tension, temperature, and entropy of mixing. Common emulsion types include water-in-oil and oil-in-water. Emulsifiers help stabilize emulsions by reducing interfacial tension and protecting newly formed droplets. Emulsion stability can be improved through techniques like charge stabilization, increasing viscosity, reducing droplet size, and using emulsifier blends or polymers at the interface.