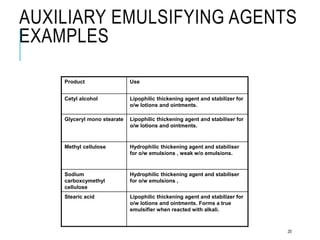
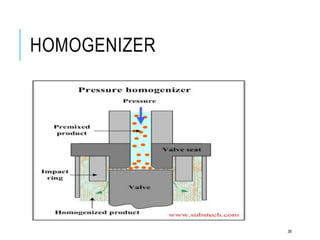
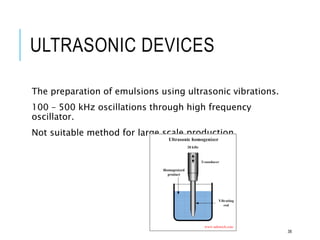
This document discusses pharmaceutical emulsions. It defines emulsion as an unstable system consisting of two immiscible liquids where one is dispersed as globules in the other. There are several types of emulsions including simple, multiple, micro, and nanoemulsions. The type of emulsion can be determined through tests like dilution, dye solubility, conductivity, cobalt chloride, and fluorescence. Emulsions have pharmaceutical applications for oral, parenteral, and topical products. They can increase drug solubility, bioavailability, and favor topical drug delivery. The key components of emulsions include oils, emulsifiers, preservatives, antioxidants, and viscosity modifiers.