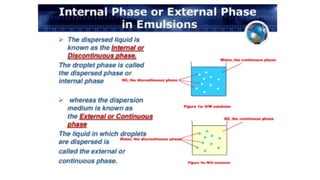
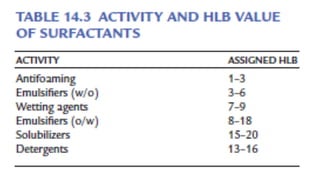
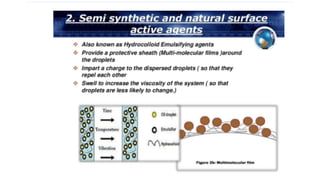
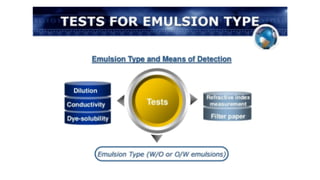
The document discusses biphasic systems, specifically emulsions, explaining their purpose and the theories behind emulsification such as surface tension theory, oriented-wedge theory, and inter-facial film theory. It outlines various methods for preparing emulsions, including the continental and English methods, while detailing the role of surfactants and their hydrophilic-lipophilic balance (HLB) in stabilizing emulsions. Additionally, it provides examples of oral and topical emulsions, emphasizing their formulations and packaging requirements.