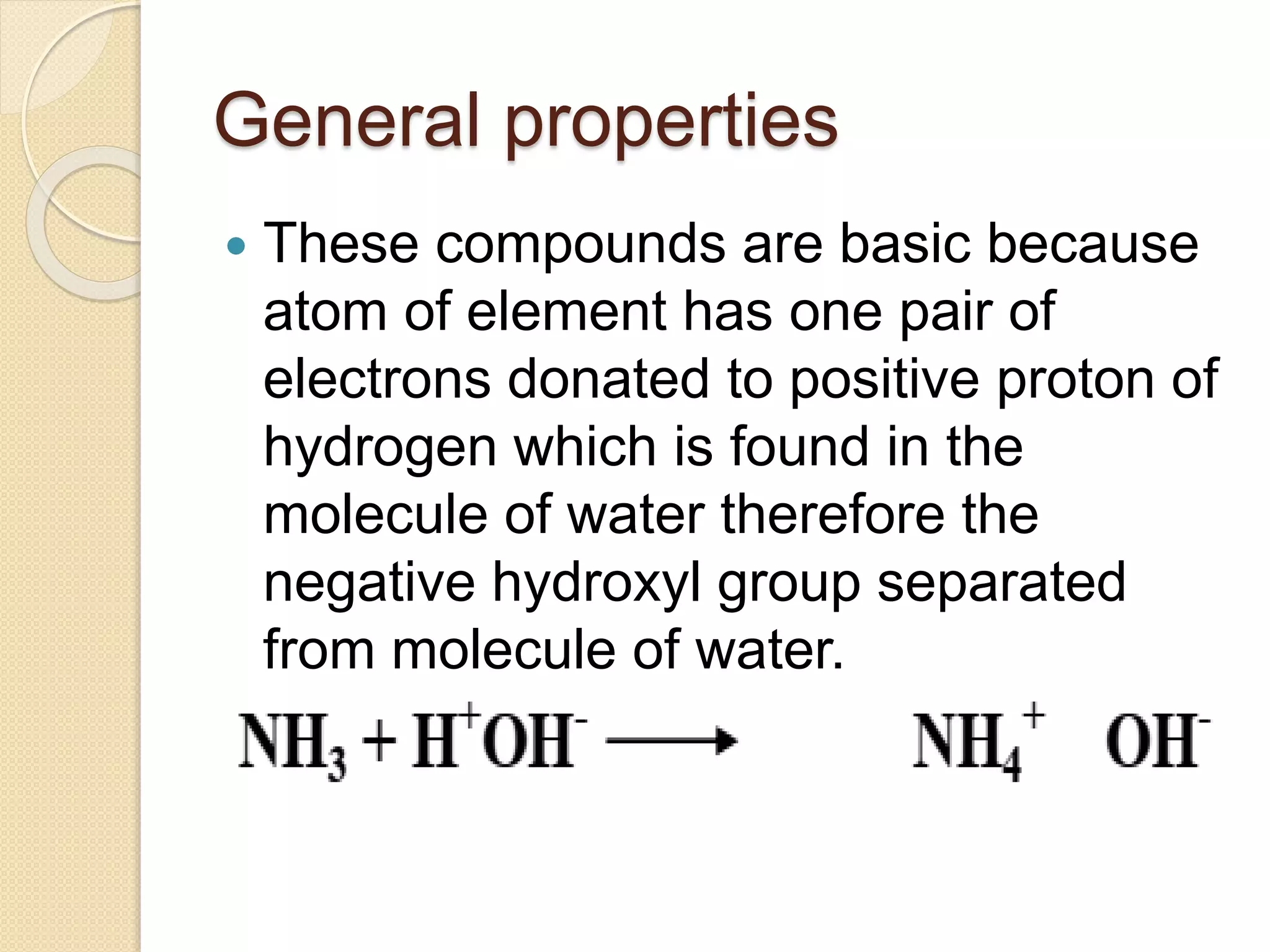
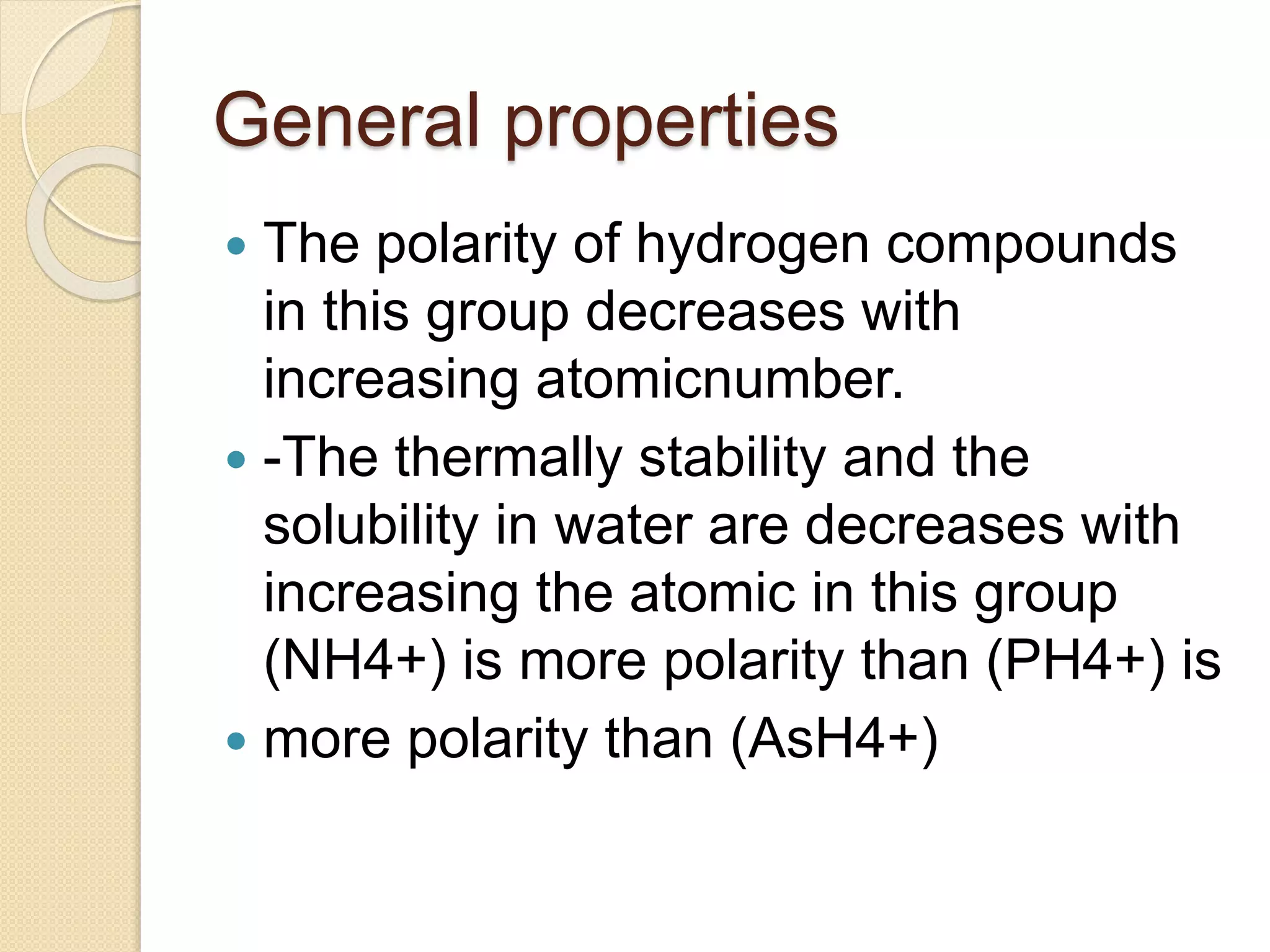
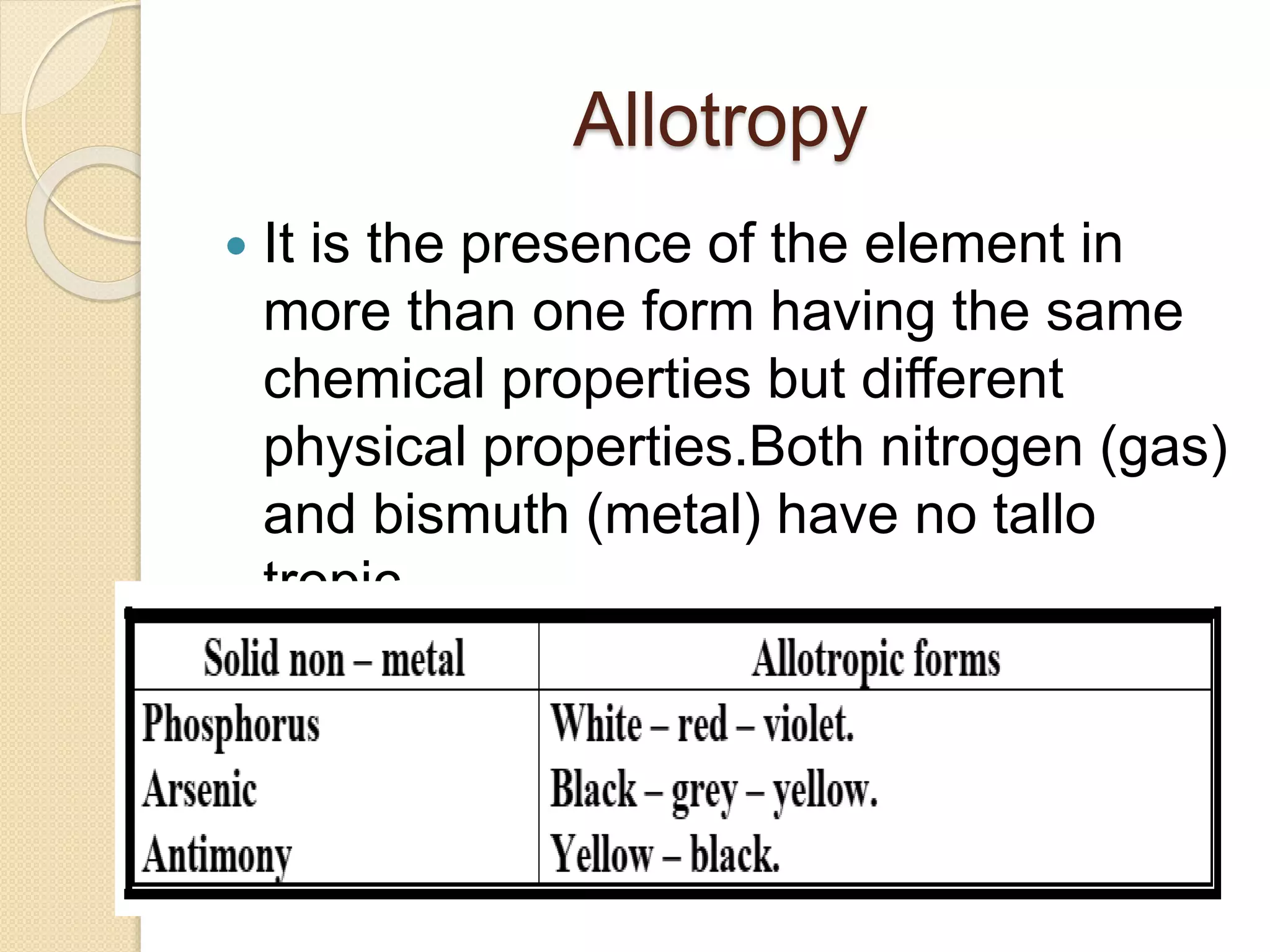
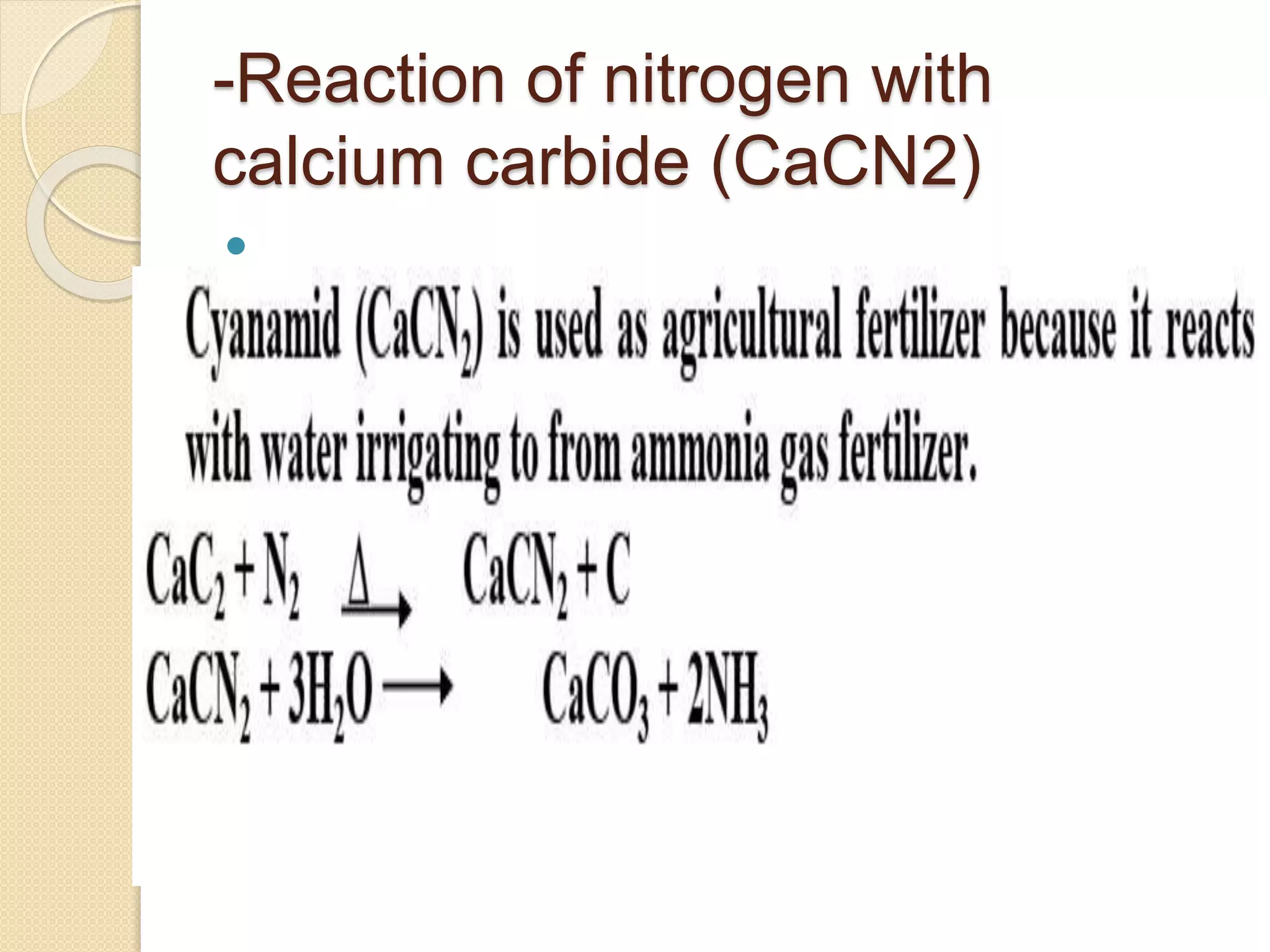
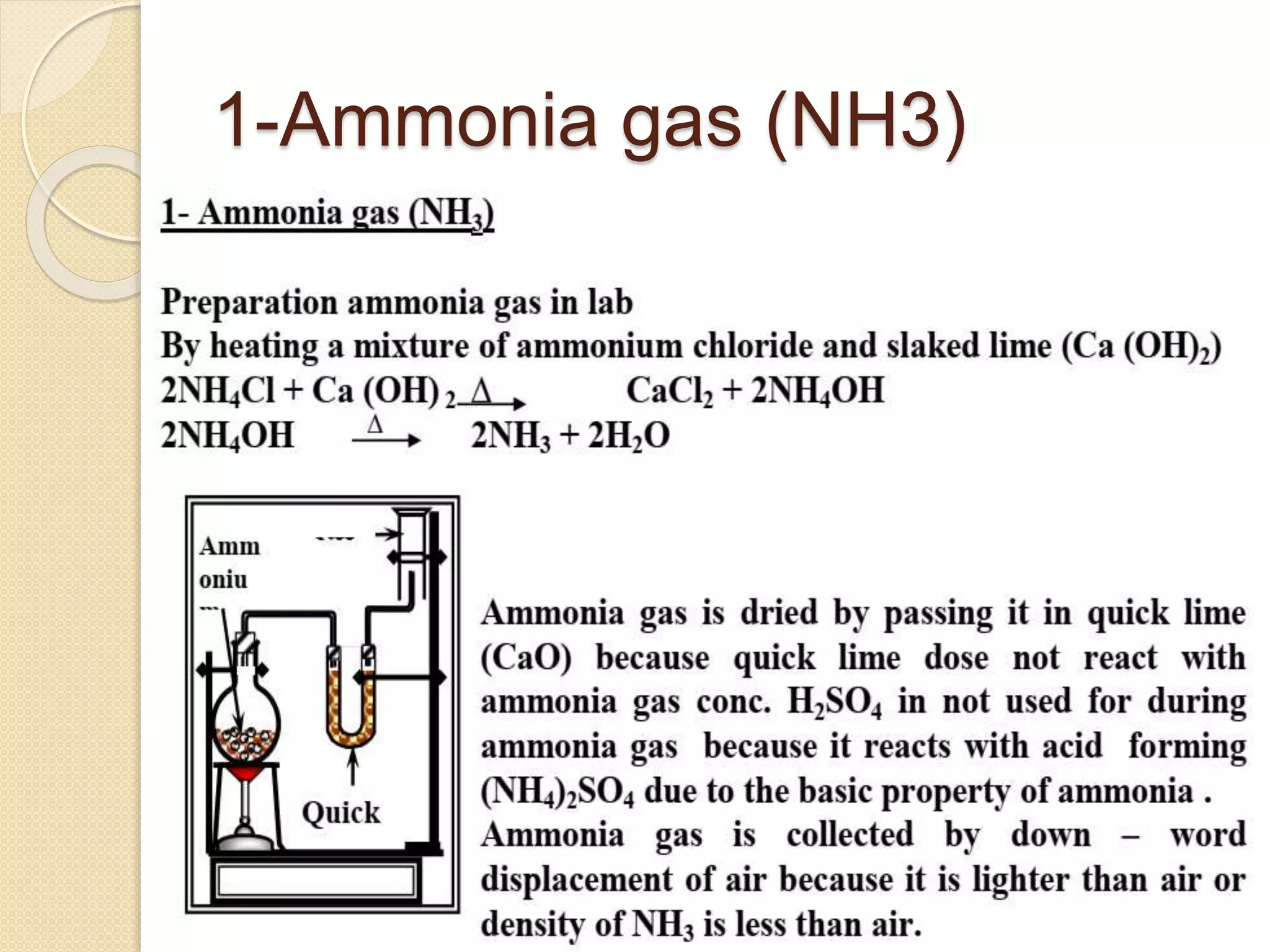
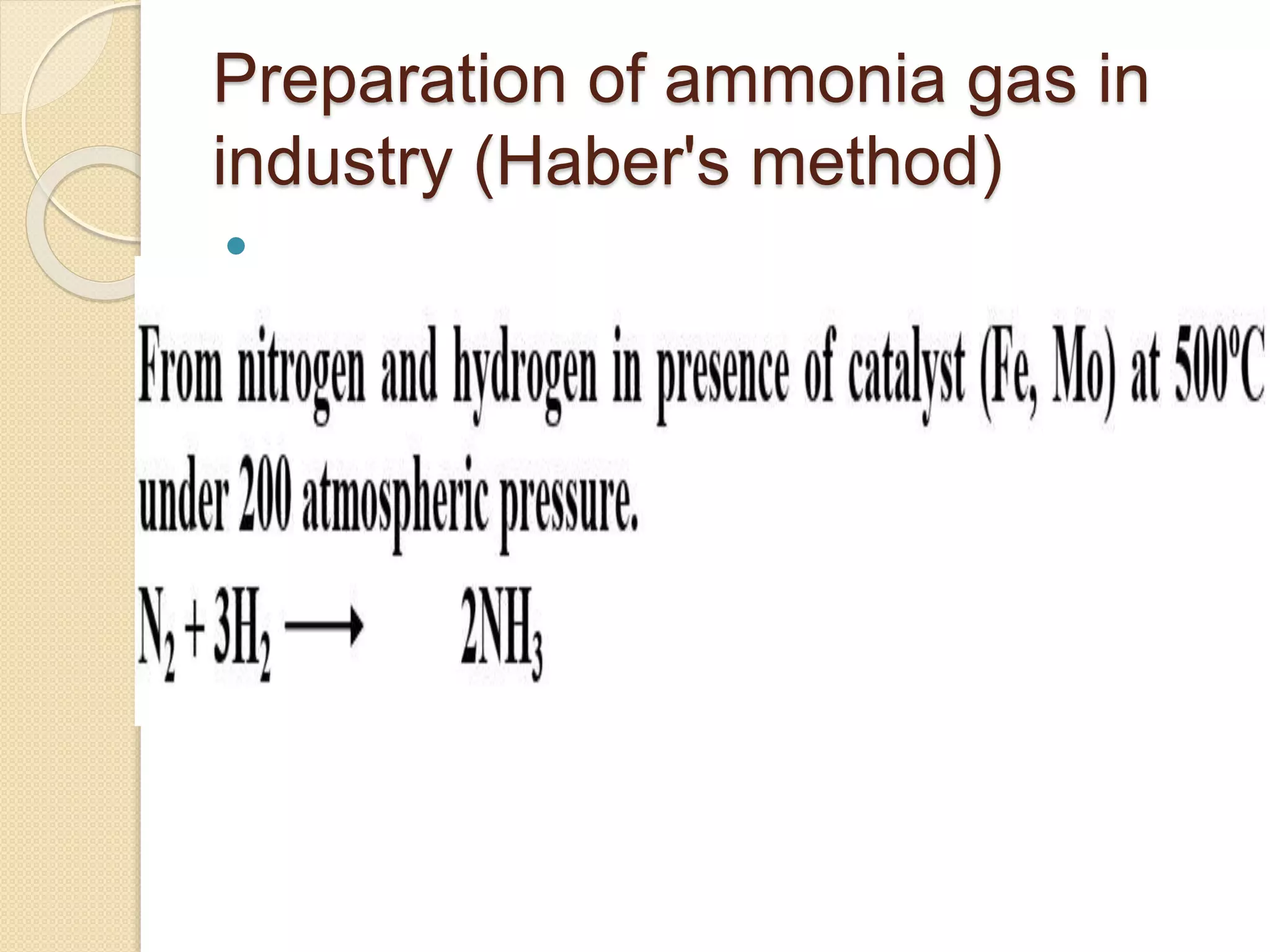
This document discusses the elements in Group 5A of the periodic table, including their general properties. Nitrogen is a diatomic gas that makes up 80% of air. Phosphorus forms calcium phosphate and reacts with hydrogen to form phosphine. Bismuth forms a crystal lattice and is a weak conductor of electricity. Elements in this group can gain electrons through covalent sharing to reach stability, form acidic or basic oxides, and hydrides like ammonia and arsine that can form coordinate bonds. Properties like polarity decrease with increasing atomic number. Nitrogen and bismuth exhibit no allotropy while nitrogen reacts with calcium carbide to form ammonia gas, an important industrial product made via the Haber process


![General properties
-Oxidation number: Elements of group
[5–A] have several oxidation numbers
because they gain electrons from 1 to
3 through covalent
sharing or electrons from 1 to 5
electron and reach to the stability state](https://image.slidesharecdn.com/elementsofgroup5a-230503060311-d3fceaaf/75/Elements-of-group-5A-pptx-3-2048.jpg)